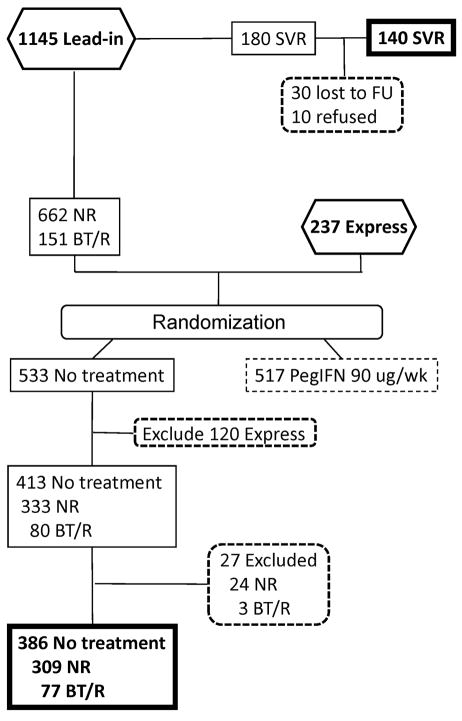
Figure 1.
Flow-diagram of HALT-C subjects included in the current analysis. Of the 1145 patients entering the lead-in phase and receiving treatment with peginterferon alfa-2a 180 ug/week and ribavirin 1–1.2 grams/day, 180 achieved an SVR. Of these, 140 participated in the current study (SVR group). 813 patients from the HALT-C lead-in phase and 237 patients who were non-responders or relapsers to peginterferon/ribavirin given outside of the HALT-C Trial (Express) were randomized to either no treatment (control arm) or to peginterferon alfa-2a 90ug/week for 3.5 years, and then followed without treatment. For the current analysis, we excluded all patients randomized to receive peginterferon 90 ug/week in order to eliminate a possible effect of long-term interferon on outcomes. Among the 533 patients randomized to no treatment, 120 patients from the Express arm were excluded because data on whether the patients were NR or BT/R was not available. Of the remaining 333 nonresponders in the control arm, 24 were eliminated because they were not followed after randomization (17), received interferon treatment after randomization (6) or had undetectable HCV RNA during follow-up (1). Three of the 80 patients with BT/R were eliminated because they were not followed after randomization (2) or received treatment with peginterferon (1).