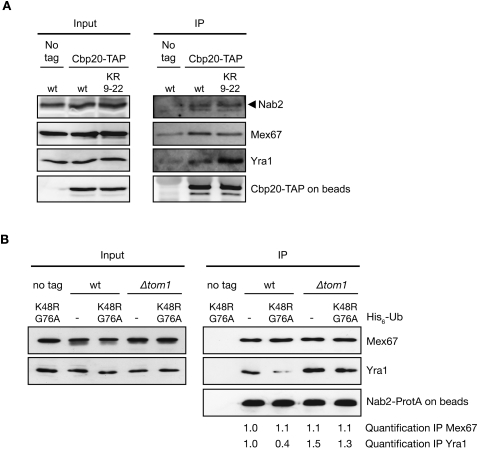
Figure 4.
Yra1 ubiquitination reduces interaction with mRNP components. (A) The Yra1 KR9–22 lysine mutant increases the association of Yra1 with nuclear mRNPs. Total extracts (Input) or IgG-Sepharose-purified extracts (IP) from Cbp20-TAP-tagged or nontagged (No tag) strains expressing wild-type or KR9–22 mutant Yra1 were analyzed by Western blotting with anti-Nab2, anti-Mex67, and anti-HA antibodies to detect Yra1. Bead-associated Cbp20-TAP was revealed with an anti-ProtA antibody, and served as control for equal amounts of mRNP pulled down. (B) Tom1-mediated ubiquitination of Yra1 induces Yra1 release from Nab2–Mex67 complexes. Total extracts prepared from Nab2 ProtA-tagged or nontagged (no tag) strains transformed with UbK48R,G76A or empty vector (−) were purified on IgG beads. Western blotting of total extract (Input) or high-salt eluted proteins (IP) were examined with antibodies against Mex67, Yra1, and ProtA. Quantification of the signal observed in the Nab2-ProtA immunoprecipitate for Mex67 and Yra1 (normalized to the signal in wild-type cells transformed with empty vector) is indicated below.