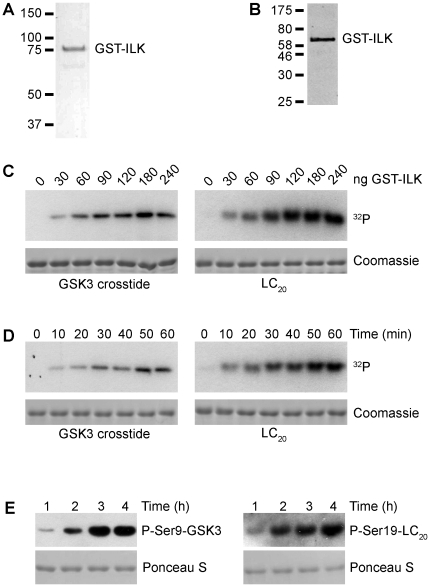
Figure 1. Purified GST-ILK is an active serine kinase.
(A) Coomassie blue-stained polyacrylamide gel showing a single protein band at approximately 78 kDa, the predicted molecular weight of the GST-ILK fusion protein, prepared as described in Materials and Methods . (B) Western blot analysis using antibodies specific for ILK confirm the identity of the 78 kDa band. (C) Analysis of the concentration dependence of the protein kinase activity of GST-ILK using GSK-3 crosstide and LC20 as substrates. Experiments were carried out using standard kinase reaction conditions (described in Materials and Methods ) in the presence of 10 mM MgCl2. Representative autoradiographs (upper panels) show the amount of 32P-phosphate incorporation with increasing concentrations of GST-ILK. Coomassie stains of SDS-gels (lower panels) depict equal loading of substrate into the kinase reaction. (D) Time course of the protein kinase activity of GST-ILK. Reactions were performed as described in C using GSK3 crosstide and LC20 as substrates. The amount of ILK was held constant at 30 ng. (E) Phosphorylation of Ser9 on GSK3 crosstide and Ser19 on LC20. Each substrate was incubated with 150 ng of GST-ILK in 25 µl of kinase reaction buffer (see Materials and Methods ) for the indicated times in the presence of 500 µM cold ATP. Western blot analysis was carried out using phospho-specific antibodies as described in Materials and Methods . Ponceau S stains of membranes are provided as loading controls.