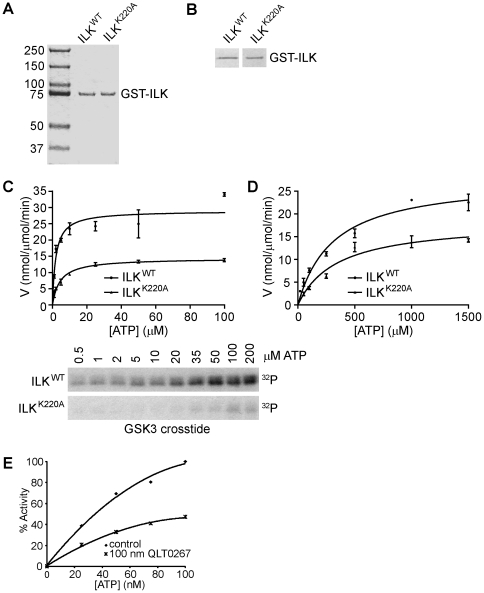
Figure 5. ILK activity is reduced by mutation of K220 and by a specific pharmacological inhibitor, QLT0267, and is required for Ser9 phosphorylation of GSK-3β in vivo.
(A) Coomassie-stained SDS-gel showing equal amounts of wild-type GST-ILK (ILKWT) and mutant GST-ILK (ILKK220A) prepared as described in Materials and Methods . (B) Western blot analysis using antibodies specific for ILK to confirm the identities of ILKWT and ILKK220A. Western blot analysis confirmed the presence of equal amounts of protein for both constructs. (C) Top, plot of reaction velocity with increasing ATP concentrations for ILKWT and ILKK220A in 5 mM MnCl2. Bottom, autoradiographic images demonstrating ILK kinase activity for ILKWT and ILKK220A with increasing ATP concentrations. Reactions were carried out for 30 min using 30 ng ILK and GSK3 crosstide as the substrate. (D) Plot of reaction velocity with increasing ATP concentrations for ILKWT and ILKK220A in 10 mM MgCl2. Reaction conditions were as described in C. (E) Kinase activity of GST-ILK in the presence of 100 nM QLT0267, a specific small molecule inhibitor of ILK. Reactions were carried out for 30 min using 30 ng ILK with GSK3 as the substrate.