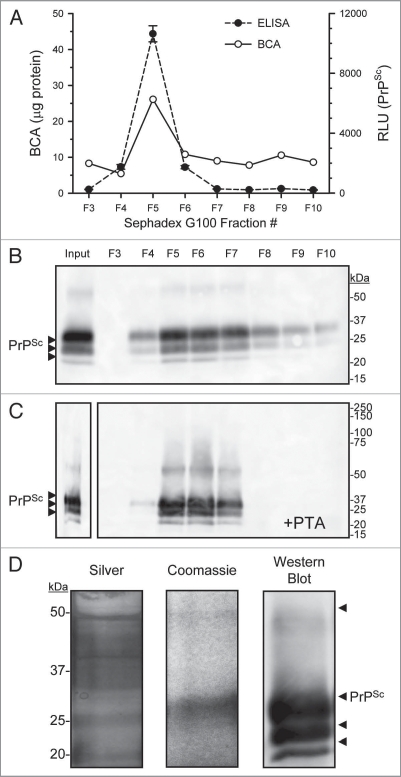
Figure 4.
Concentration and purification of prion by PTA and size exclusion chromatography. Enriched PrPSc DRM fractions were treated with proteinase-K then proteins PTA precipitated, the solubalized (PrPSc DRM-PK-PTA) material was then fractionated by size exclusion chromatography (Sephadex G100). Protein concentration of fractions was determined by BCA assay (A; solid line) and PrPSc detection by ELISA (A; dashed line). A small protein peak was observe in fraction #5 that corresponded to the void fraction of the column (proteins >100 kDa) which also contained the majority of detectable PrPSc. Western blot detection of G100 fractionated PrPSc DRM-PK-PTA-G100 showed a major band in the void fraction #5 (B). A second PTA protein precipitation following G100 fractionation recovered detectable PrPSc in the void fractions (C). Evaluation of the purified PrPSc DRMPK-PTA-G100-PTA material by silver and Coomassie stain (D; left and middle part respectively) showed detectable PrPSc protein at the expected molecular weight that corresponded to PrPSc detection by Western blot (D; right part).