Fig. 3.
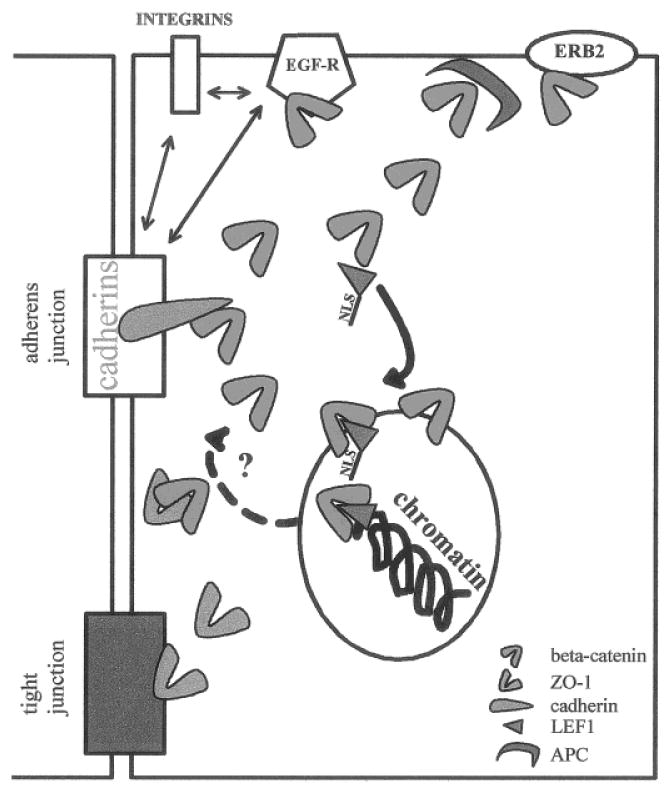
Molecular equilibrium. CMS proteins are engaged in a number of interactions with different cellular components. The relative abundance of the binding partners of CMS proteins regulates the molecular equilibrium, which in turn affects cellular function. For example, β-catenin can interact with cadherins (light blue), ZO-1 (green), APC (dark blue), EGF receptor (EGF-R), and erb-2 at the cell membrane. Moreover, cadherin/catenin, EGF-R, β-1-integrin and α6-β4 integrins interact with each other. β-Catenin binding with transcription factor LEF-1(brown) and its translocation to the nucleus, depends on the existence of a free pool of β-catenin; it may also be influenced by the amount of LEF-1 available. Other factors may contribute to the shift of the molecular equilibrium, like the interaction of plakoglobin with β-catenin partners, and Wnt-1 expression which influences the degradation process associated with the formation of APC/β-catenin complexes. The presence GSK3 β and Axin that interact with APC/β-catenin complexes may also influence the molecular equilibrium. The return of β-catenin from the nucleus (dotted arrow) to interact with cell membrane binding partners, upon reception of specific signals, remains to be demonstrated.
