Fig. 4.
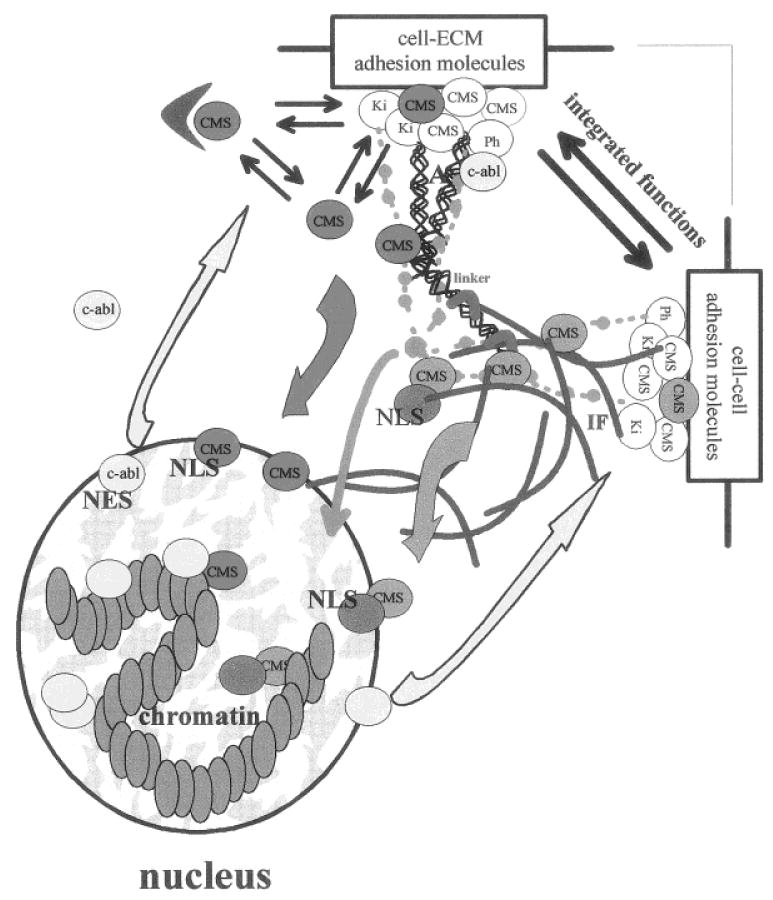
Compartmental equilibrium and dynamic reciprocity. Nuclear translocation of CMS proteins (red and orange) modifies the balance of these proteins between the membrane skeleton compartment and the nuclear compartment and shifts their molecular equilibrium toward an increase of interactions with nuclear targets. Translocating CMS proteins can be considered as structural signal transducers that act as mediators of cell-cell and cell-ECM signaling, along with biochemical cascades (light green) possibly superimposed on signaling via tension generated through actin (A) and intermediate filament (IF) networks. CMS proteins could translocate into the nucleus on their own (red) and bind to nuclear proteins (yellow), or they could travel with a carrier (dark green). The transfer of information from the nucleus to the cell membrane includes the synthesis of membrane skeleton, cell membrane, and ECM components, it may also involve feedback reactions to generated tensional force (not represented). The translocation of nuclear CMS proteins back to the membrane skeleton may also participate in inside/out signaling. Similarly, proteins primarily located in the nucleus could travel to the cell membrane (yellow), as shown with c-abl. We propose that nuclear structural proteins involved in supramolecular organization of the nucleus also may travel to the cell membrane. The balance between these interactive signaling pathways represents the dynamic reciprocity that governs cellular and tissue behavior.
