Figure 3.
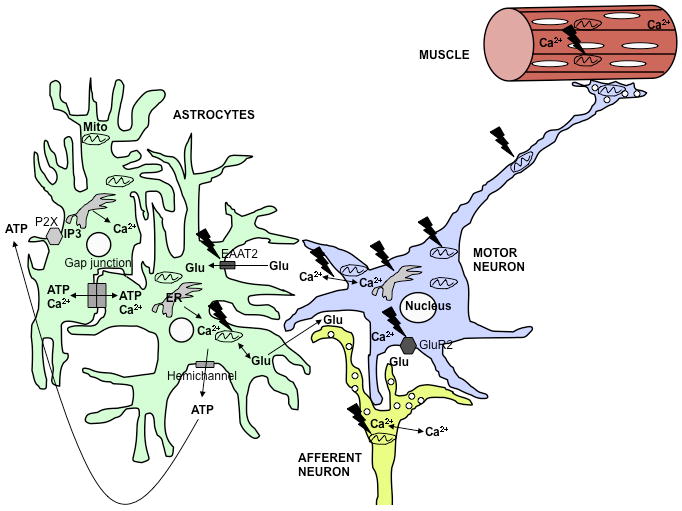
Involvement of calcium dysregulation in ALS.
In the ALS spinal cord, multiple cell types, including MNs and astrocytes, are involved in the disease. The black jagged symbols indicate potential sites of mutant SOD1 toxicity. MNs are particularly vulnerable to increased cytosolic calcium due to low levels of calcium binding proteins and enhanced permeability to calcium through AMPA receptors that contain few GluR2 subunits. Calcium uptake defects mitochondria can cause extended intracellular calcium exposure. In MNs, this may contribute to excitotoxicity, impaired mitochondrial transport, permeability transition, and apoptosis. Mitochondrial bioenergetic dysfunction contributes to increased intracellular calcium, thereby propagating a vicious cycle of calcium dysregulation and cellular damage. Additionally, defective mitochondrial calcium uptake in afferent neurons or astrocytes may result in excessive glutamate release in excitatory synapses. ALS astrocytes have low glutamate transporter (EAAT2) expression, which exacerbates glutamate excitotoxicity. Increased intracellular calcium in astrocytes can also result in excessive propagation of intercellular calcium waves, either through gap junctions or through opening of hemichannels. The resulting extracellular release of modulatory molecules, such as ATP, induces intracellular calcium rise through activation of metabotropic receptors on neighboring cells, and propagates the wave. Furthermore, muscle mitochondrial calcium handling defects can also play a role in muscle degeneration.
