Table 2.
CuBr-catalyzed regioselective diamination of dienesa
| entry | substrate (1) | product (4) | yield (%)b |
|---|---|---|---|
| 1 |
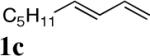
|
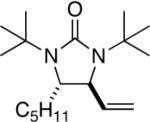
|
97 (96:4)c |
| 2 |
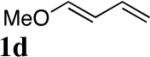
|
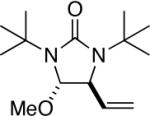
|
95 |
| 3 |
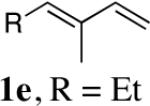
|
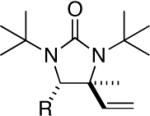
|
81 |
| 4 | 1f,R = Ph | 92 (97:3)c | |
| 5 |
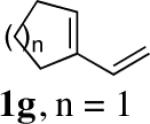
|
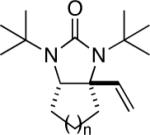
|
95 |
| 6 | 1h,n = 2 | 99 | |
| 7 | 1i,n = 3 | 94 | |
| 8 |
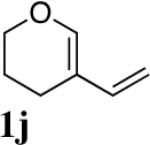
|
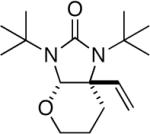
|
85 |
| 9 |
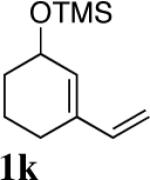
|
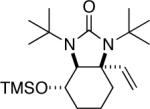
|
95 (97:3)c,d |
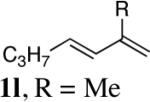
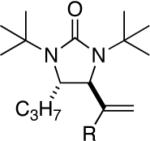
| 10 |
|
|
98 |
| 11 | 1m,R = Ph | 96 (89:11)c | |
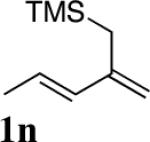
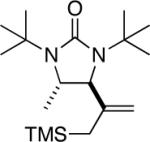
| 12 |
|
|
94 |
| 13 |
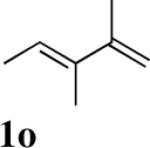
|
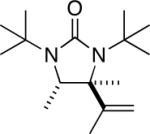
|
90 |
| 14 |
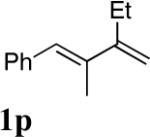
|
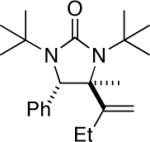
|
95 |
All reactions were carried out with olefin 1 (0.20 mmol), di-tert-butyldiaziridinone (2) (0.22 mmol), CuBr (0.010 mmol) in CDCl3 (0.4 mL) under Ar with vigorous stirring at 0 °C for 20 h unless otherwise stated. For entry 1, 1c (0.19 mmol) was used. For entry 2, olefin 1d (0.25 mmol, E:Z = 15.7:1, E isomer: 0.24 mmol) and 2 (0.2 mmol) were used. For entry 7, the reaction was carried out on 0.40 mmol scale. For entry 9, CuBr (0.020 mmol) was used.
Isolated yield based on 1 except for that of entry 2 which was based on 2.
The ratio of 4 to 3 was determined by analysis of 1H NMR spectrum of the crude reaction mixture.
The stereochemistry was tentatively assigned based upon sterics.
