Fig 1. Extrinsic and intrinsic apoptotic pathways.
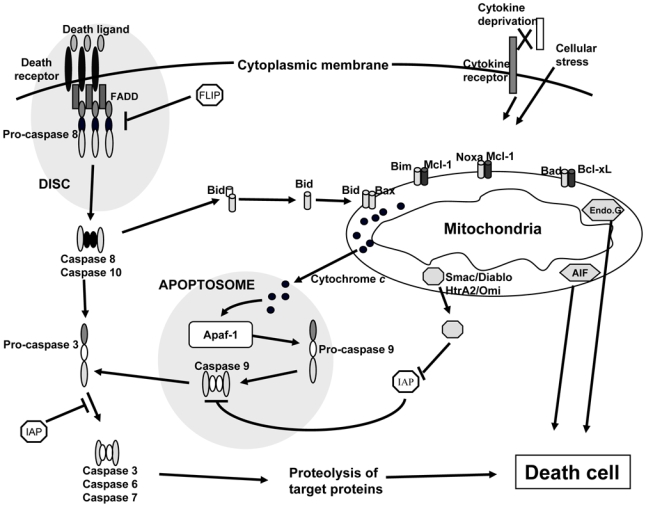
In the extrinsic apoptotic pathway, an activation of death receptors by their ligands induces the recruitment of FADD and the recruitment and activation of an initiator caspase, i.e. caspase 8. This multiprotein complex is called the “DISC”. In turn, caspase 8 activates an effector caspase, caspase 3, and consecutively the induced cellular apoptosis. Caspase 8 is likewise able to cleave cytosolic Bid, which is translocated to the mitochondrial membrane and thus constitutes a link between extrinsic and intrinsic apoptotic pathways. In the later, various proapoptotic stimuli induce critical modifications on the mitochondrial outer membrane in the balance between pro and antiapoptotic Bcl-2 family members, inducing the membrane-permeabilization and release of cytochrome c into the cytosol. Cytochrome c activates Apaf-1 to constitute a macromolecular complex (apoptosome) which recruits and activates caspase 9 leading to caspase 3 activation and to the proteolysis of target proteins and finally to apoptosis. AIF and endonuclease G are apoptogenic proteins released in the cytosol as cytochrome c but which induce a caspase-independent apoptosis.
