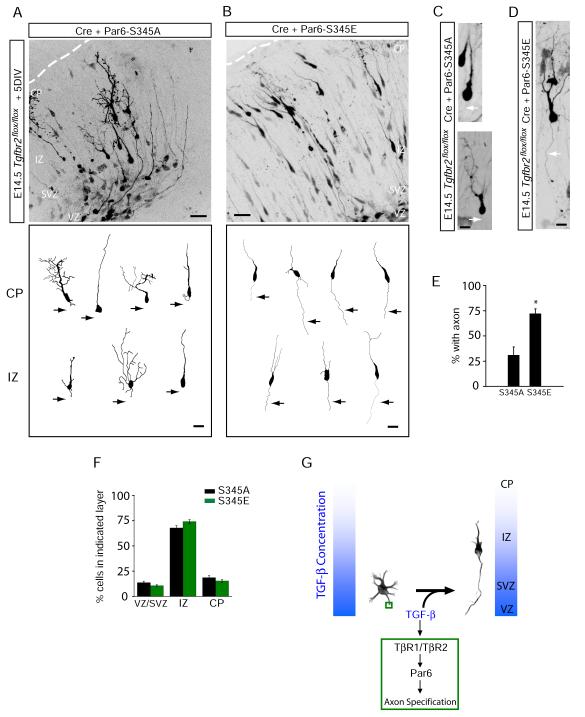
Figure 7.
Phosphomimetic Par6-S345E Restores Axons in TβR2 KO Neurons in Vivo
(A) Neuronal progenitors in E15 Tgfbr2flox/flox embryos were electroporated with Cre plus Par6-S345A and examined five days later. The bottom panel shows representative traces of neurons in the intermediate zone (IZ) and cortical plate (CP).
(B) A phosphomimetic mutant of Par6 rescues axon specification. Neuronal progenitors in E15 Tgfbr2flox/flox embryos were electroporated with Cre plus Par6-S345E. The bottom panel shows representative traces of neurons in the IZ and CP. For (A) and (B), scale bars are 50 μm for top panels and 20 μm for bottom panels.
(C) Morphology of migrating TβR2-KO neurons expressing Par6-S345A, showing the absence of an axon (arrows). Scale bar, 22 μm.
(D) Morphology of a migrating TβR2-KO neuron expressing Par6-S345E showing the presence of a trailing axon (arrows). Scale bar, 22 μm.
(E) Data represent means ± SEM of the percent neurons with an axon. Experiments were averages from at 3-4 embryos. Par6-S345A, n = 89 cells; Par6-S345E, n = 84; *p<0.05, Student’s t-test.
(F) Quantitative analysis of migration defects in TβR2-KO neuron expressing Par6-S345A or Par6-S345E. Par6-S345E did not rescue migration defects caused by the loss of TGF-β signaling. Compare to Figure S2D. Results represent experiments from at least 4 embryos. n = 2357, 4598 cells for Par6-S345A and Par6-S345E, respectively. See Supplemental Methods for details.
(G) A model for TGF-β–dependent axon specification in developing neocortex. VZ, ventricular zone; IZ, intermediate zone; CP, cortical plate.