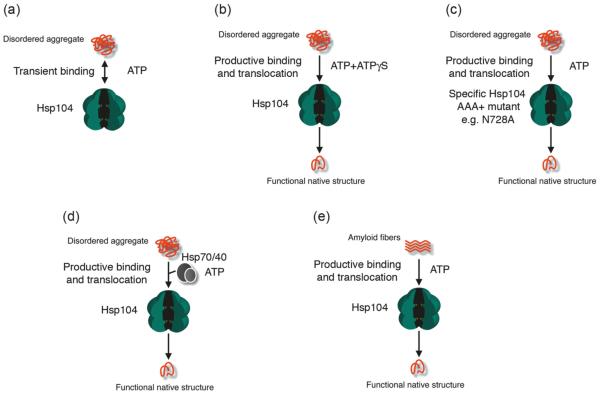
Fig. 2.
Co-ordination of Hsp104 ATPase activity is required for protein disaggregation. (a) In the presence of ATP, Hsp104 engages denatured aggregates too transiently to initiate disaggregation. (b) In the presence of specific mixtures of ATP and ATPγS (e.g., 3 ATP : 1 ATPγS), Hsp104 is able to productively couple substrate binding to translocation and disaggregation. Only specific ratios of ATP and ATPγS are effective, and pure ATPγS inhibits activity (Doyle et al. 2007b). (c) In the presence of ATP, specific Hsp104 mutants with reduced ATPase activity at NBD2, such as N728A or K620T, can productively couple substrate binding to translocation and disaggregation (Doyle et al. 2007b). Despite this innate ability to disaggregate some disordered aggregates, these mutants fail to disaggregate amyloid and are unable to synergize with the Hsp70 chaperone system and are defective in vivo (Doyle et al. 2007b; Hattendorf and Lindquist 2002; Shorter and Lindquist 2004). (d) In the presence of ATP, Hsp70 and Hsp40 ensure productive substrate binding by Hsp104, which leads to translocation and disaggregation. (e) In the presence of ATP, Hsp104 is able to bind and disaggregate amyloid substrates.