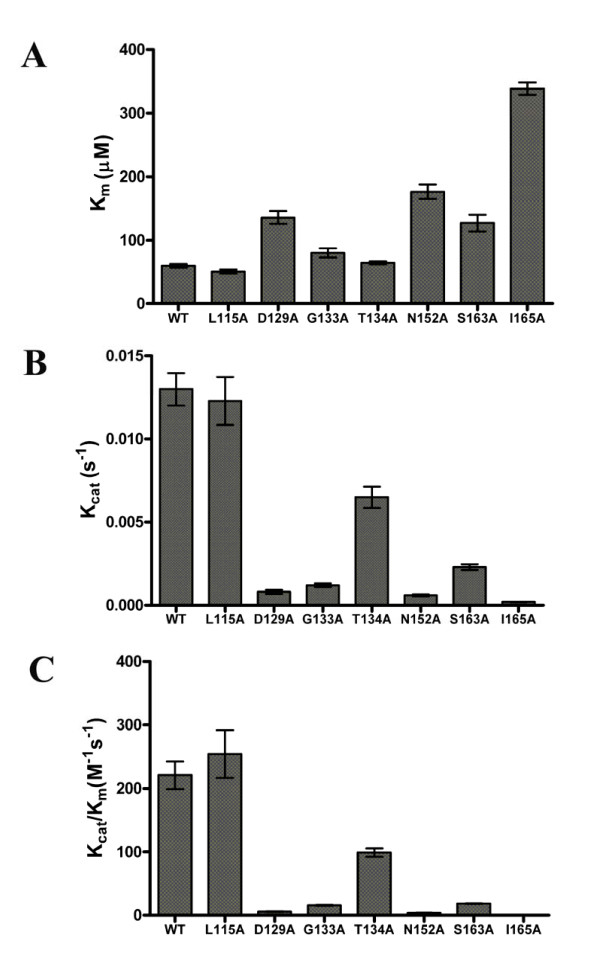
Figure 4.
Presentation of kinetic parameters for samples of NS2B(H)-NS3pro wild-type and mutant derivatives. Samples were assayed by using fluorescence emission from cleavage of the peptide substrate GRR-amc at 37°C as described under Methods. The bar graph shows a comparison of numerical constants obtained for Km (panel A), kcat (panel B) and catalytic efficiency kcat/Km (panel C) for the wild-type protein NS2B(H)-NS3pro and active site mutant proteins L115A, D129A, G133A, T134A, N152A, S163A and I165A. Samples of Y150A and G151A were inactive in the enzyme assay and a 23-fold increase in enzyme concentration did not result in detectable activity. Data represent the mean of triplicate measurements and error bars are indicated.