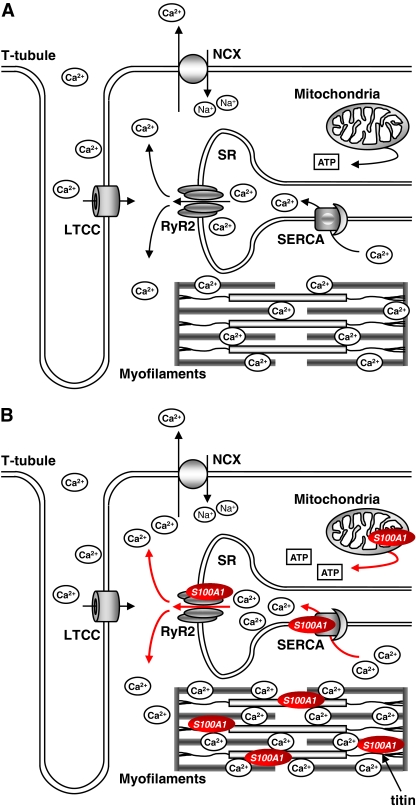
Fig. 3.
Proposed model for S100A1 molecular interactions in cardiomyocytes. a During excitation–contraction coupling, voltage-dependent opening of the L-type Ca2+ channel (LTCC) leads to a transsarcolemmal Ca2+ entry that triggers sarcoplasmic reticulum (SR) Ca2+ release via ryanodine receptor 2 (RyR2). Increased sarcoplasmic Ca2+ levels eventually result in myofilament cross-bridge cycling and mechanical force development. During diastole, resequestration of sarcoplasmic Ca2+ into the SR is operated by the SR Ca2+ ATPase (SERCA). To keep steady-state conditions, the NCX excerts transsarcolemmal Ca2+ extrusion in balance with LTCC-conducted Ca2+ entry. b S100A1 interacts with both RyR2 and SERCA and is present at mitochondria and myofilaments. Increased S100A1 protein levels enhance systolic SR Ca2+ release via RyR2 without influencing LTCC-conveyed Ca2+ entry. Augmented systolic SR Ca2+ release is compensated by elevated SERCA activity and diminished SR Ca2+ leakage during diastole, altogether improving SR Ca2+ cycling and mechanical force generation. Additionally, S100A1 interaction with mitochondrial F1-ATPase leads to increased ATP synthesis and enhanced energy supply. Finally, myofilament pre-contractile passive tension is reduced by S100A1 interference with titin–actin interplay in the sarcomere