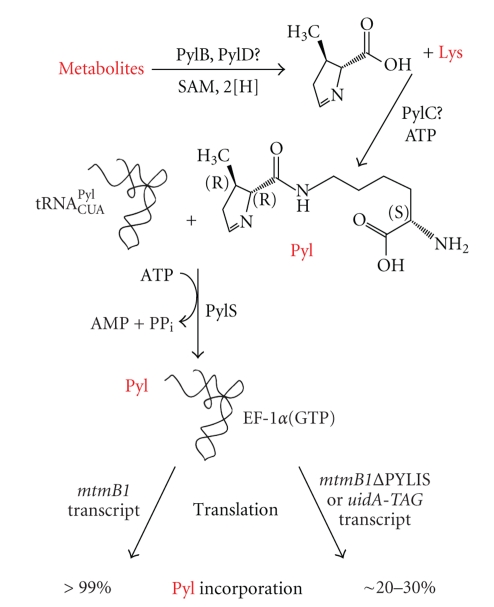
Figure 4.
Schematic representation of pyrrolysine incorporation into protein. The pylB, pylC, and pylD genes have been shown to enable pyrrolysine biosynthesis in E. coli, but their exact roles are unknown. A conceptual and speculative scheme is shown which is keeping with the activities of proteins in their respective proteins families. Lysine is likely to form the acyl of pyrrolysine, but may also be coupled to an early precursor which subsequently cyclizes after amide bond formation. Pyrrolysine is given entrance to the genetic code by PylS in Archaea, the equivalent in Bacteria are the products of the split gene pylSc and pylSn. The direct formation of pyl-tRNAPyl is likely followed by binding to the elongation factor used by the canonical amino acids, that is, EF-1α in Archaea. The common bacterial elongation factor EF-Tu can bind charged tRNAPyl both in vivo and in vitro. The recognition of pyl-tRNAPyl by non-specialized elongation factors underlies the relatively high level of UAG translation in a reporter gene such as uidA with an introduced amber codon in organisms having tRNAPyl, PylS, and pyrrolysine or an analog to charge tRNAPyl. The PYLIS is not essential for this level of translation, as shown by replacement of PYLIS in mtmB1, but may enhance UAG translation to some extent. This effect is unlikely to require the structure formed by PYLIS. See text for further details.