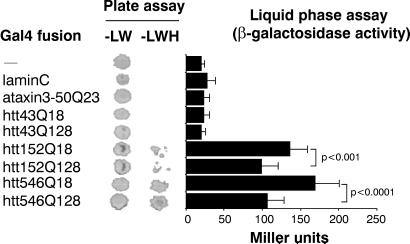
Figure 1.
Interaction of C. elegans ZK1127.9(187–758) with human htt in yeast two-hybrid tests. The first 43 (htt43), 152 (htt152), or 546 (htt546) amino acids of normal (18 Gln, Q18) or mutated (128 Gln, Q128) htt were tested for interaction with ZK1127.9(187–758). In plate assays with selective medium lacking Leu (L), Trp (W), and His (H), only normal and mutated htt152 and htt546 bound to ZK1127.9(187–758). In liquid-phase assays, mutated htt152 and htt546 bound weakly to ZK1127.9(187–758) compared with normal htt152 and htt546, respectively (ANOVA). No interaction was detected with the Gal4-binding domain alone (−), a random bait protein (lamin C), the first 50 aa of normal ataxin3 with 23 consecutive glutamines (ataxin3–50Q23), and N-terminal htt lacking the Pro-rich region (htt43Q18 and htt43Q128). Results for β-galactosidase activity are the mean ± SD (n = 15).