Figure 3.
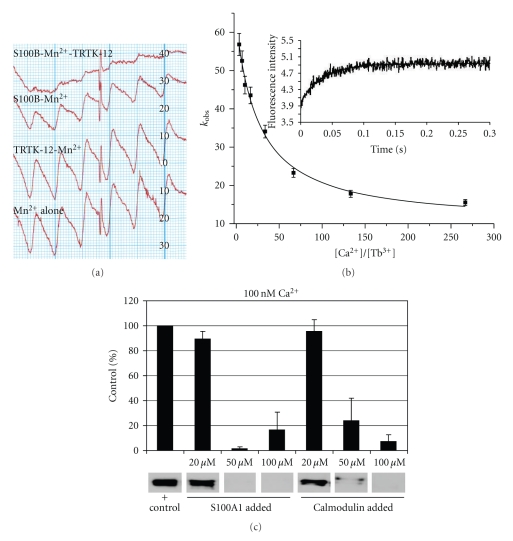
Metal ion and target binding properties of S100 proteins. (a) Binding studies with Mn2+ were completed since it is a good probe of the high affinity Ca2+ binding site on S100B (EF2) [113, 135]. Free Mn2+ was measured by electron paramagnetic resonance (EPR) in the absence and presence of S100B (+/− target peptide TRTK12) [34]. In all four traces, total [Mn2+] is identical (80 μM) with the bottom trace (4th trace) showing the signal for total [Mn2+]. TRTK12 alone (1 mM) has no effect on the EPR signal (3rd trace), whereas, the addition of S100B (65 μM) binds Mn2+ and reduces free [Mn2+] (2nd trace). The addition of the same amount of S100B (65 μM) plus TRTK12 (1 mM, top trace) has the least free [Mn2+] and indicates that TRTK12 binding to S100B-Mn2+ enhances Mn2+ binding (compare traces 1 and 2). A similar effect was observed for SBi1 (unpublished) and for p53367-388 [109]. As for p53367-388 and SBi1, TRTK12 increased the affinity of S100B for Ca2+ in competition studies with Mn2+ and via stopped-flow kinetic measurements of Cakoff as monitored in competition with Tb3+. (b) Plot of the decrease in kobs as a function of [Ca2+]/[Tb3+] as used to determine the off rate of Ca2+ from the 2nd EF-hand (EF2, Cakoff). The kobs values at each [Ca2+]/[Tb3+] ratio were calculated from kinetic traces of stopped-flow experiments where Tb3+ (syringe C) is mixed with S100B at varying Ca2+ concentrations (syringe A) and [Tb3+] signal is monitored as a function of time (λ ex = 230 nm, λ em = 545 nm). A Cakoff of 60 ± 8/sec was calculated from these experiments with S100B alone. When either TRTK12 or SBi1 is present, then the calculated Cakoff value for S100B is reduced to 5 ± 3/sec similar to that found for p53367-388 [109]. These studies demonstrated that TRTK12, p53367-388, or SBi1 increased the affinity of S100B for Ca2+ at least in part by decreasing Cakoff. In (c), S100A1 was found to bind the full length ryanodine receptor (RyR) at 100 nM free calcium. Specifically, S100A1 competed full-length RyR1 away from agarose-linked CaM beads as judged by a decreased RyR1 band in an anti-RyR Western blot. Free CaM, a positive control, also competed the RyR away from CaM-linked beads [60, 67].