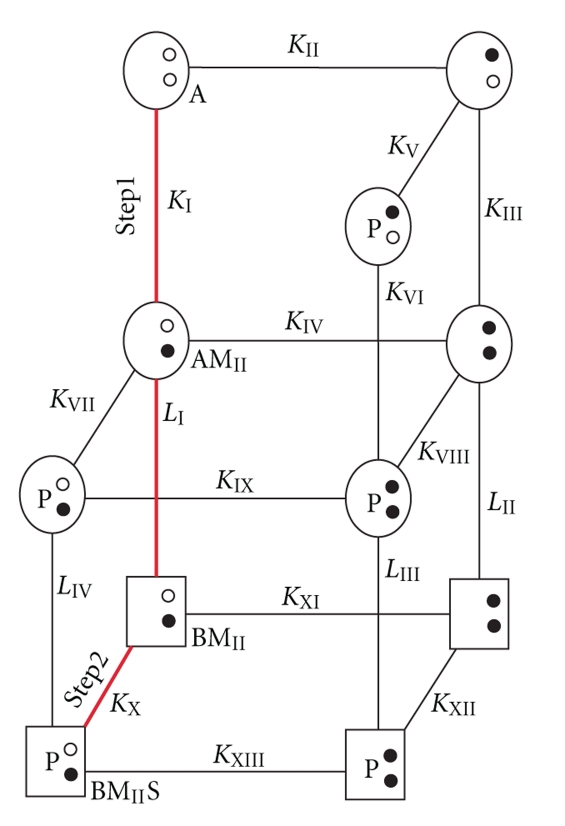
Figure 5.
Models for Ca2+-binding and then target-binding to an S100 protein. (Top) A model for the Ca2+-dependent interaction of S100B with target proteins involves 13 equilibrium constants (KI to KXIII), 11 states, and 4 conformational changes (LI–LIV) [109]. The most highly populated states and the predominant pathway are colored red; this is due to weak Ca2+ binding in the pseudo-EF-hand (site I), which greatly simplifies this model (see Scheme 1). Specifically, the binding of Ca2+ to the pseudo- and typical EF-hand in each S100B subunit is described by six states (A, AMI, AMII, AMIMII, BMI, and BMII), five equilibrium constants (KI = [A][M]/[AMI], KII = [A][M]/[AMII], KIII = [AMI][M]/[AMI,II], KIV = [AMII][M]/[AMI,II], and KXI = [BMII][M]/[BMI,II], two conformational changes (LI: AMII↔ BMII, LII: AMI,II↔ BMI,II) with corresponding rate constants, respectively, where A = S100B prior to the 90° reorientation of helix three of S100B, B = S100B after 90° reorientation of helix three, MI = a Ca2+ ion bound to EF-hand I (pseudo-EF-hand), MII = a Ca2+ ion bound to EF-hand II (typical EF-hand), MI,II = Ca2+ ions bound to EF-hand I and EF-hand II. Upon the addition of p53 or another target (S), the model expands to 11 possible states, 13 dissociation constants, and four possible conformational changes. Whether additional equilibriums occur (KXIV, KXV, and KXVI) is considered in Scheme 1. (Bottom) In a second model (Scheme 1), state A is defined as the “closed” conformation observed in the apo-state (Figure 2), and state B is after a 90° reorientation of helix 3 termed the “open” conformation. In black, are states hypothesized to be populated. [A-MII]‡ and [B-MII]‡ represent short-lived intermediates, and L1 is the Ca2+-dependent conformational change involving helix 3 of S100B upon binding Ca2+ (Figure 2). Based on NMR relaxation rate data from the PI's lab [136], KXIV highly favors state A. States are also considered via KXV and KXVI which result in B* states that represent an ensemble of dynamic structures, of which, only a subset fully coordinate Ca2+ as observed in X-ray structures [9]. It is hypothesized that KXV favors the B*MII state(s), whereas, KXVI favors B-MII-S, explaining the apparent increase in Ca2+-binding affinity using equilibrium binding measurements that monitor free [metal ion] (Figure 3).