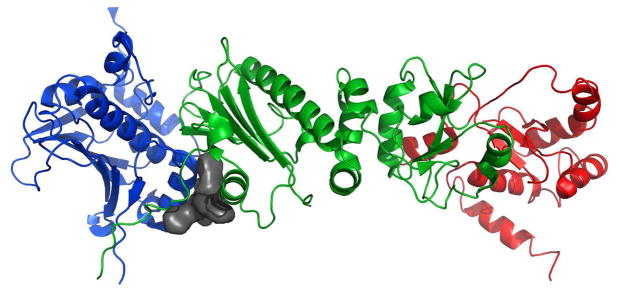
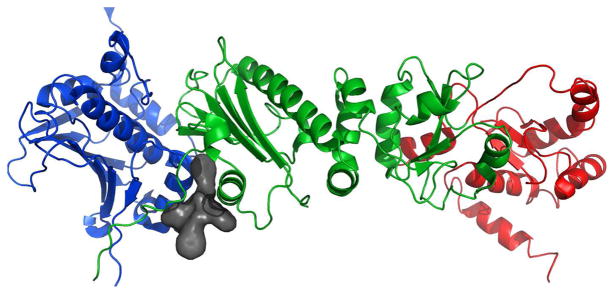
Figure 13.
a) Full-length Hsp90 monomer with Compound 19 in the predicted binding site between the N-Middle domain. Blue, green and red are for the N, middle and C terminal domains respectively. 19 (space-filling, grey) is bound to the region between the N and Middle domains of full-length yeast Hsp90. This potent derivative adopts a conformation that fits well inside the binding pocket. b) 20 (space-filling, grey) is bound to the region between the N and Middle domains. Note how the differences in conformation prevent this non-potent derivative from inserting deep into the pocket, exposing a majority of the structure to solvent. The proposed binding site on Hsp90 was identified using AutoLigand correlated with pull-down assay results and the San A derivative binding mode was determined using AutoDock4.2. Molecular graphics were prepared using PyMol.