Table 3.
Comparisons of α selectivities for various donor-acceptor pairs
| Entry | Donor | Acceptor | Product | Conditions | Yield[a] | Ratio[b] (α/β) |
|---|---|---|---|---|---|---|
| 1 | 2 |
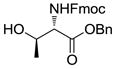 9 |
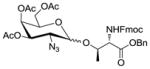 1031 |
TMSOTf rt | 79% | 8.2 |
| 2 | 2 | 9 | 10 | HClO4-SiO2 rt | 81% | 19 |
| 3 | 2 |
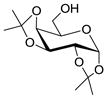 11 |
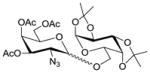 1232 |
TMSOTf 0°C | 96% | 6.1 |
| 4 | 2 | 11 | 12 | HClO4-SiO2 0°C | 94% | 8.1 |
| 5 | 2 |
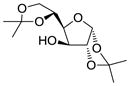 13 |
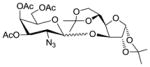 14 |
TMSOTf 0°C | 84% | 13 |
| 6 | 2 | 13 | 14 | HClO4-SiO2 0°C | 86% | 32 |
| 7 |
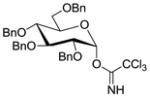 15 |
11 |
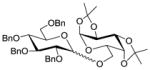 1633 |
TMSOTf 0°C | 93% | 3.0 |
| 8 | 15 | 11 | 16 | HClO4-SiO2 0°C | 90% | 3.0 |
| 9 | 15 | 11 | 16 | HClO4-SiO2 rt | 85% | 4.0 |
| 10 | 15 | 13 |
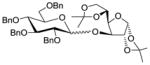 1734 |
TMSOTf 0°C | 84% | 4.0 |
| 11 | 15 | 13 | 17 | HClO4-SiO2 0°C | 88% | 9.3 |
| 12 |
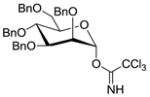 18 |
11 |
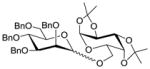 1935 |
TMSOTf 0°C | 85% | 3.0 |
| 13 | 18 | 11 | 19 | HClO4-SiO2 0°C | 88% | 10 |
Combined yield of both anomers after purification
Determined by 1H NMR spectroscopy
