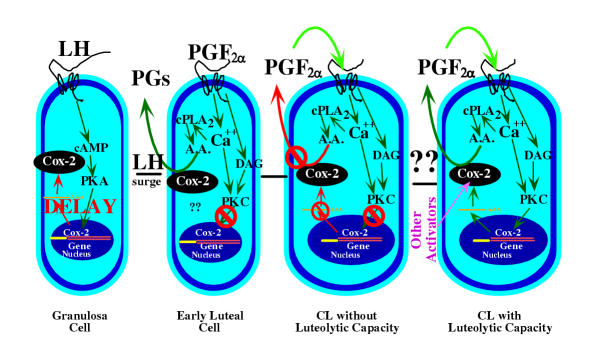
Figure 2.
A model for the regulation of PG production during different stages of luteal differentiation. In the granulosa cell of the preovulatory follicle there is very low PG production and low expression of Cox-2. The LH surge induces Cox-2 expression through the protein kinase A (PKA) pathway but with a delay in expression depending upon the species [83]. In the early luteal cell there is high PG production that is stimulated by pathways that have not yet been defined. It is also possible that high Cox-2 protein has been left after the dramatic induction of Cox-2 after the LH surge. In the early luteal cell and in the CL without luteolytic capacity (these 2 stages may overlap), there are PGF2α receptors but PGF2α does not stimulate increased intraluteal PG production (shown by red lines). In addition, PGF2α does not induce other activators of PG production, such as decreased progesterone secretion, increased endothelin-1 production, or increased cytokine production. Unknown mechanisms cause the CL to acquire luteolytic capacity. After acquisition of luteolytic capacity, treatment with PGF2α increases intraluteal PG production. Activation of cytosolic phospholipase A2 (cPLA2) by increased free intracellular calcium concentrations provides arachidonic acid (A.A.) substrate to the induced Cox-2 enzyme. Although not shown, these events are likely to be localized to the nuclear membrane. Intraluteal PGF2α production activates an autoamplification loop in the mature CL due to PGF2α-induced Cox-2 expression and PGF2α induction of other activators of Cox-2 expression.