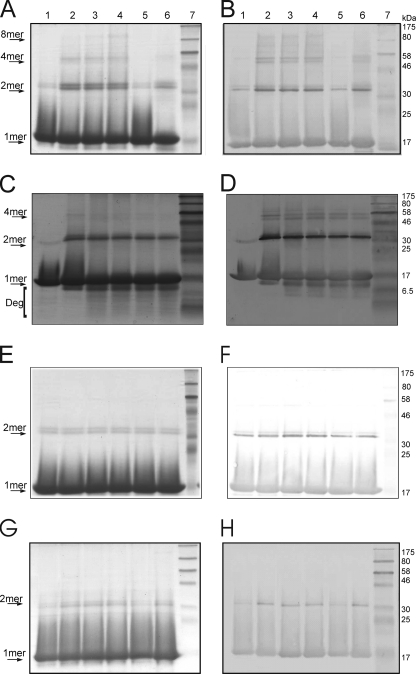
FIGURE 4.
Iron-mediated cross-linking of HbpS. HbpS WT (A and B) and HbpSHis28Ala (G and H) proteins were treated with FeCl2 (100 μm, lane 2; 250 μm, lane 3; 500 μm, lanes 4–6) and 5 mm DTT. Recombinant catalase was added before start of treatment (lane 5). Additional H2O2 was added after treatment (lane 6). All treated samples as well as the native one (lane 1) were subjected to 12% SDS-PAGE. C and D, WT-treated sample (with FeCl2 and DTT; lane 2) was further incubated with H2O2 for different times (10 min, lane 3; 30 min, lane 4; 60 min, lane 5; 120 min lane 6). All treated samples as well as the native one (lane 1) were subjected to 15% SDS-PAGE. E and F, HbpS was treated with FeCl2 (lane 1), FeCl3 (lane 2), or DTT (lane 3). Aliquots of each of these samples were incubated with H2O2 (H2O2 + FeCl2, lane 4; H2O2 + FeCl3, lane 5; H2O2 +DTT, lane 6). All samples were subjected to 12% SDS-PAGE. After electrophoresis, proteins were analyzed by Coomassie staining (A, C, E, and G) or by immunoblotting using anti-HbpS antibodies (B, D, F, and H). Arrows indicate positions for the monomeric (1mer), dimeric (2mer), tetrameric (4mer), or octameric (8mer) HbpS forms. The degradation products are also indicated (Deg). The approximate sizes of prestained protein markers are also shown (lane 7).