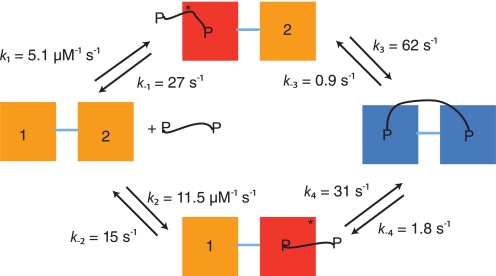
FIGURE 6.
Kinetic reaction scheme for the interaction between PSD-95 PDZ1–2 and a dimeric peptide. The initial association and dissociation rate constants (k1, k2, k−1, k−2) in the scheme were obtained from experiments with monomeric peptide, but are fully consistent with the dimeric peptide binding data. The reverse rate constants (k−3, k−4) were measured from displacement kinetics of the PDZ tandem constructs and dimeric peptide. In the displacement reaction from wild type PDZ1–2 tandem, only the slowest intramolecular koff can be determined (0.9 s−1); the rate constant of 1.8 s−1 is inferred from, first, the intermolecular koff values between monomeric peptide and single PDZ domains and, second, from the ratio of intramolecular koff values from dimeric peptide and Trp mutant tandems PDZ1*–2 and PDZ1–2* (not shown). In both cases the off-rate constant from PDZ1 is roughly two times that of PDZ2. The intramolecular forward rate constant k3 was determined from the dependence of the slow step for PDZ1–2* and dimeric peptide (Fig. 5F, kobsmax ≈ k3 = 62 s−1), as described in the Results section. Finally, k4 was calculated as 31 s−1, given that the product of rate constants on one half of the scheme must equal that of the other half.