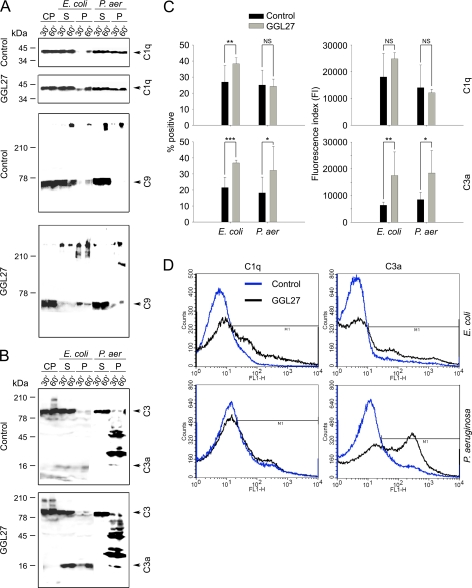
FIGURE 6.
The C-terminal TFPI peptide GGL27 enhances C1q, C3a, and MAC binding to E. coli. A, E. coli ATCC 25922 and P. aeruginosa 15159 bacteria were washed, resuspended, and incubated with citrate plasma either alone or supplemented with GGL27 (at 3 μm) for 30 min or 1 h at 37 °C. The bacterial cells were collected and washed with PBS, and bound proteins and corresponding supernatants were subjected to Tris-Tricine SDS-PAGE under reducing conditions, followed by immunoblotting with antibodies recognizing C1q or C5b-9. CP, citrate plasma; S, supernatant or unbound bacteria; P, pellet or material bound to bacterial cells. B, as in A, but antibodies against the C3a were used (25). In C: left panel, comparison of the mean proportion of bacteria positive for C1q/C3a binding in citrate plasma (control, black columns) and in plasma supplemented with GGL27 (gray columns). Right panel, comparative degree of C1q and C3a binding to E. coli and P. aeruginosa strains, expressed as means of the fluorescence index (FI; proportion of bacteria positive for C1q/C3a multiplied by the mean intensity of C1q/C3a binding). (*, p < 0.05; **, p < 0.01; and ***, p < 0.001, t test). D, examples of flow cytometry histograms of C1q/C3a binding to E. coli and P. aeruginosa in citrate plasma and in plasma supplemented with GGL27.