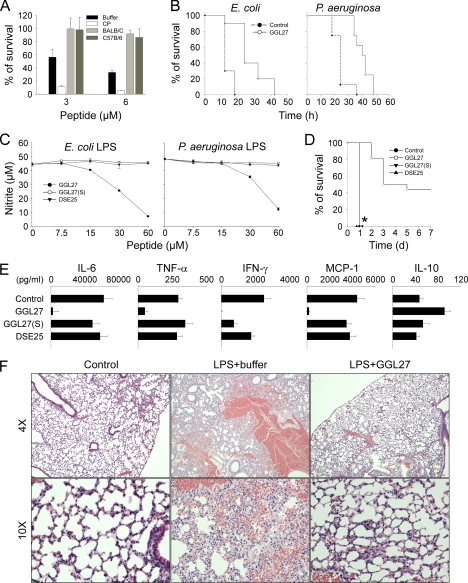
FIGURE 8.
In vitro and in vivo effects of GGL27. A, 2 × 106 cfu/ml E. coli were incubated in 50 μl with GGL27 peptide at the indicated concentrations in 10 mm Tris, pH 7.4, buffer (Buffer), or in 0.15 m NaCl, 10 mm Tris, pH 7.4, containing 20% human citrate plasma (CP), or in the same buffer but with mice citrate plasma (Balb/c or C57B/6) (n = 3, ±S.D. is indicated). B, the GGL27 peptide prolongs survival in E. coli- and P. aeruginosa-infected mice. Mice were injected intraperitoneally with E. coli or P. aeruginosa bacteria. In the E. coli infection model GGL27 (200 μg) or buffer alone was injected intraperitoneally after 30 min (n = 10 in each group, p < 0.001, Kaplan-Meier Survival Analysis Log-Rank test). In the P. aeruginosa infection model GGL27 (500 μg) or buffer alone was injected subcutaneously after 1 h (n = 8 in each group, p < 0.001, Kaplan-Meier Survival Analysis Log-Rank test). C, GGL27 inhibits NO production. RAW264.7 mouse macrophages were stimulated with LPS from E. coli (left panel) or P. aeruginosa (right panel) in presence of GGL27 or the two control peptides GGL27(S) and DSE25 at the indicated concentrations. The difference between GGL27 and the control peptides is statistically significant (p < 0.01). D, GGL27 significantly increases survival in LPS-induced shock. Mice were injected with E. coli LPS followed by intraperitoneal administration of GGL27 (500 μg). Buffer and the peptides GGL27(S) and DSE25 (500 μg) served as controls. Survival was followed for 7 days (GGL27, n = 16; buffer, n = 15; GGL27(S), n = 8; DSE25, n = 8; the difference between GGL27 and buffer, or the control peptides, is significant, p < 0.01, Kaplan-Meier Survival Analysis Log-Rank test). *, indicates that the lines for GGL27(S), DSE25, and buffer are overlaid. E, GGL27 attenuates pro-inflammatory cytokines. In a separate experiment, mice were sacrificed 20 h after intraperitoneal injection of LPS followed by treatment as above with GGL27 (500 μg), buffer, or the control peptides GGL27(S) or DSE25, and the indicated cytokines were analyzed in blood (control, n = 8; GGL27, n = 12; GGL27(S), n = 7; DSE25; n = 8). In all cases, the difference between GGL27-treated animals and buffer was significant. p values for the respective cytokines are IL-6, 0.0023; TNF-α, 0.0018; IFN-γ, 0.0002; MCP-1, 0.0002; and IL-10, 0.0023. There was a significant difference between controls and GGL27(S) with respect to IFN-γ. F, lungs were analyzed 20 h after intraperitoneal LPS injection followed by treatment with GGL27 (500 μg) or buffer. Histochemical analysis shows marked attenuation of inflammatory changes in GGL27-treated lungs (a representative lung section is shown).