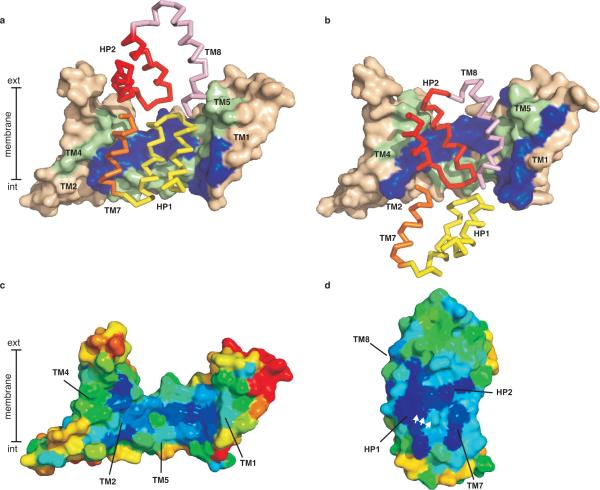
Figure 3. Domain interaction surfaces.
Trimerization domains of WT GltPh (a) and GltPh-55C/364CHg (b) are shown in surface representation and coloured wheat. Residues involved in domain contacts, identified by ProFace server47, are coloured blue (TM1 and 2) and green (TM4 and 5). Interacting structural elements of the transport domain are shown in ribbon representation: HP1 (yellow) and TM7 (orange) in WT GltPh, and HP2 (red) and TM8 (pink) in GltPh-55C/364CHg. Surface representation of the trimerization (c) and transport (d) domains coloured according to evolutionary conservation. Dark blue and red correspond to the highly conserved and variable residues, respectively. The interacting surfaces are facing the viewer and the white arrows mark the highly conserved serine-rich signature motif in HP1. Conservation scores were calculated using Consurf server48 and 212 SLC1 sequences with less than 60 % identity harvested from Pfam database49 and aligned in ClustalW250.