Figure 1.
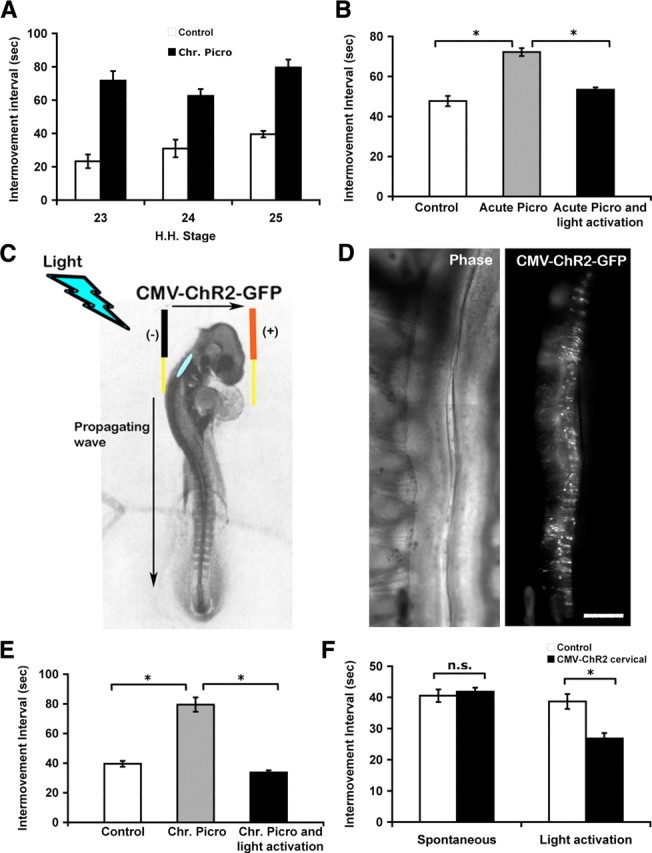
Intervals between episodes of axial movement activity in control, picrotoxin-treated, and picrotoxin together with light activation by ChR2-treated embryos. A, Bar graph of intervals between in ovo movements of control and chronic picrotoxin-treated embryos at St 23, 24, and 25. Chronic picrotoxin treatment slowed the frequency of bursting activity. B, Bar graphs of the intermovement intervals in ovo quantified at St 25 in control embryos, embryos acutely treated with picrotoxin at St 25, and embryos acutely treated with picrotoxin and that had been stimulated with brief flashes of light every 40 s (*p < 0.025). C, Schematic showing the approximate location (blue oval) in the upper cervical spinal cord, where the ChR2–eGFP construct was injected in St 18 chick embryos. Even though the construct was electroporated into one side of the cervical spinal cord, light activation was able to elicit a propagating wave into the lumbar region. D, ChR2–eGFP expression was detected in the spinal cord of St 25 chick. Left, Phase contrast picture of spinal cord whole mount showing cord and DRGs on the left side. Right, Same field as left, showing ChR2 expression on one side of the cord. Rostral is up. Scale bar, 200 μm. E, Intermovement intervals in control, chronic picrotoxin-treated embryos, and embryos treated with picrotoxin but activated with light by ChR2 assessed at St 25. Chronic picrotoxin treatment slowed the frequency of bursting activity. Light activation by ChR2 in addition to chronic picrotoxin treatment maintained the frequency of bursting activity similar to control values (*p < 0.025). F, Intermovement intervals were also quantified in control embryos and those electroporated with ChR2. Unless stimulated with brief flashes of light at twice the normal frequency, electroporation of ChR2 had no significant effect on the intermovement interval at St 25 (*p < 0.025). Chr. Picro, Chronic picrotoxin treatment.
