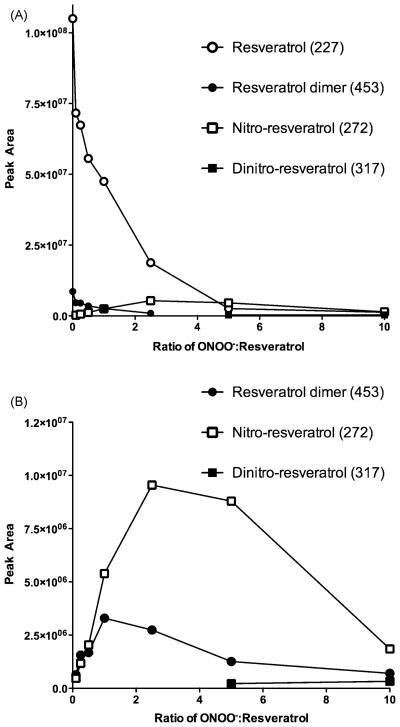
Fig. 4. LC/MS/MS analysis of resveratrol-peroxynitrite reaction products.
Increasing ratios of ONOO−:resveratrol resulted in loss of resveratrol and the appearance of isomeric mono- and di-nitroresveratrol and resveratrol dimers (panel A). Analysis of product ions (panel B) showed that as the ONOO−:resveratrol ratios increased, peak areas of the major product ion transitions for the resveratrol dimers and the mono-nitroresveratrol isomers increased. The peaks for mono-nitroresveratrol isomers (m/z 272 → 225) and resveratrol dimers (m/z 453 → 265) were observed at all ONOO− concentration ratios. The peak for di-nitroresveratrol isomers ((m/z 317 → 254) ) was observed only for ONOO−:resveratrol ratios above 5. Data are representative of three separate experiments.