Abstract
ME1071, a maleic acid derivative, is a novel specific inhibitor for metallo-β-lactamases (MBL). In this study, the potentiation of ME1071 in combination with several β-lactams was evaluated using MBL-producing Pseudomonas aeruginosa isolates. The rates of susceptibility of MBL producers to carbapenems (imipenem, biapenem, and doripenem) and ceftazidime were increased by 8 to 27% in the presence of 32 μg/ml of ME1071. The corresponding resistance rates were decreased by 13 to 46%, respectively. On the other hand, ME1071 showed weaker or no potentiation with non-MBL producers. The Ki value of ME1071 for IMP-1 was 0.4 μM, significantly lower than the Km values of carbapenems for the IMP-1 enzyme. On the other hand, the Ki value of ME1071 for VIM-2 was 120 μM, higher than the Km values of carbapenems for the VIM-2 enzyme. Results of this study indicate that ME1071 can potentiate the activity of ceftazidime and carbapenems against MBL-producing strains of P. aeruginosa.
Carbapenem use has increased during the past 2 decades. This is due, in part, to carbapenems' broad spectrum of antibacterial activity and their resistance to hydrolysis by extended-spectrum β-lactamases. However, the emergence of carbapenemases and other carbapenem resistance mechanisms is threatening this antibiotic class (18, 20). In a recent survey, 12.4% of Pseudomonas aeruginosa isolates from clinical specimens showed resistance to imipenem (8). In P. aeruginosa, carbapenem resistance has been attributed to two main mechanisms: (i) the loss of the OprD outer membrane porin channel and/or overexpression of some efflux pumps, possibly in combination with high-level production of the resident AmpC β-lactamase, and (ii) the production of carbapenem-hydrolyzing class A or class B β-lactamases (9, 18).
Class B β-lactamases are metallo-β-lactamases (MBLs) that require zinc ions for their activity (5, 18). Although clinically significant, MBLs remain rare, though their frequency has been increasing in P. aereuginosa (3, 8). Most MBLs hydrolyze most β-lactams, including carbapenems such as imipenem, meropenem, biapenem, and doripenem. In Japan, the IMP-1 subgroup is the most prevalent MBL (8, 10). In Japan, nationwide surveillance studies have reported MBL producers to represent 2 to 3% of P. aeruginosa isolates from clinical specimens (8).
The majority of MBL producers exhibit a multidrug-resistant (MDR) phenotype, including also resistance to aminoglycosides and fluoroquinolones (8, 10). For these reasons, there is interest in the administration of β-lactams together with an MBL inhibitor as therapy for infection by MBL-producing multidrug-resistant organisms.
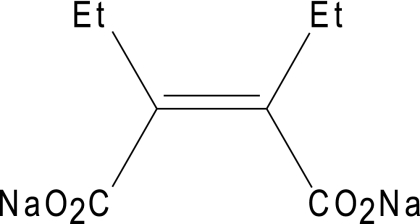
ME1071 (Fig. 1) is a novel specific inhibitor for MBLs discovered by Meiji Seika Kaisha Ltd. (Tokyo, Japan). The aim of this study was to evaluate the MBL-inhibitory activity of ME1071 by using 174 nonduplicate clinical isolates of MBL-producing P. aeruginosa and 16 nonduplicate non-MBL-producing P. aeruginosa isolates. The kinetic parameters of IMP-1 and VIM-2 for 5 carbapenems and ME1071 were also determined using purified enzymes.
FIG. 1.
Chemical structure of ME1071.
(Part of this work was presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 2007.)
MATERIALS AND METHODS
Bacterial strains.
The nonduplicate P. aeruginosa collection (190 isolates) included 166 IMP-1 MBL producers, 8 VIM-2 producers, and 16 non-MBL-producing multidrug-resistant isolates from 164 hospitals in Japan, isolated in the period 1997 to 2008. Strain identification was performed with the BD Phoenix system (Becton Dickinson, Sparks, MD) using NMIC/ID30 panels according to the manufacturer's instructions. The type of MBL was confirmed by PCR analysis according to established protocols (18, 19). The genotype of tested isolates was confirmed by repetitive-element-based PCR assays (20).
Susceptibility testing.
The MICs were determined by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (1). Commercial dry plates for antibiotic susceptibility testing of Gram-negative organisms were purchased from Eiken Chemical Co., Ltd. (Tokyo, Japan), and included the β-lactam antibiotics piperacillin, ceftazidime, aztreonam, imipenem, meropenem, biapenem, and doripenem. Cation-adjusted Mueller-Hinton broth (Becton Dickinson) was added to the plate in the presence or absence of 32 μg/ml (final concentration) of ME1071. Cultures were adjusted to an optical density of an 0.5 McFarland standard using a nephelometer and diluted 1:10 in sterile saline. Duplicate plates with and without ME1071 were inoculated in parallel with the same suspension by using an automatic MIC-2000 inoculator (Dynatech Laboratories, Inc., Alexandria, VA) so that the final inoculum was approximately 5 × 105 CFU/well. The MIC represented the lowest concentration of antibiotic that completely inhibited visible bacterial growth and was read at 16 to 18 h after inoculation. For quality control of the susceptibility testing, the following reference strains were used: Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853. The Clinical and Laboratory Standards Institute (2010) does not have interpretative criteria (susceptible, intermediate, or resistant) for biapenem or doripenem (2). For this study, the breakpoints for imipenem (2) were used for biapenem and doripenem.
Expression and purification of IMP-1 and VIM-2.
The expression and purification procedures for IMP-1 and VIM-2 β-lactamases have been described previously (4, 11, 12). Briefly, pET28a (Takara Bio Inc., Shiga, Japan) was used as an expression vector for overproducing IMP-1 and VIM-2 (4). The purified β-lactamases were stored in 50 mM HEPES (pH 7.5)-50 μM ZnSO4 buffer at −80°C until use.
Determination of kinetic parameters of MBL for carbapenems.
Kinetic parameters were determined by measuring hydrolysis rates with a Shimadzu UV-2550 spectrophotometer (Shimadzu Co., Kyoto, Japan), connected to a personal computer (7). Steady-state kinetic parameters were determined as described previously (7, 11). Each reported parameter is an average of three independent measurements. All reactions were performed in a total volume of 500 μl at 30°C. All kinetic parameters were determined by measuring the initial hydrolysis rate of the selected antibiotic and using the Michaelis-Menten equation (7). Ki for ME1071 was determined using nitrocefin as a reported substrate (4, 11, 12).
RESULTS AND DISCUSSION
Antibiotic susceptibility testing.
All study P. aeruginosa isolates were identified as clonally unrelated strains by using the repetitive PCR method (data not shown). The overall rates of carbapenem nonsusceptibility were 93% and 80% among MBL-producing and non-MBL-producing strains, respectively (Table 1). Seventy-six percent (n = 132) of MBL-producing P. aeruginosa strains and 37% (n = 6) of non-MBL-producing P. aeruginosa strains exhibited a multidrug-resistant phenotype (resistance to imipenem, ciprofloxacin, and amikacin determined by the Phoenix system).
TABLE 1.
Antibiotic susceptibility of P. aeruginosa in the presence or absence of 32 μg/ml of ME1071
| Organism (no. of isolates tested) and antibiotic | ME1071a | MIC (μg/ml) |
% Sa | % Ra | ||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||||
| MBL-producing P. aeruginosa (174) | ||||||
| Piperacillin | − | 0.5->128 | >128 | >128 | 34 | 66 |
| + | 1->128 | 128 | >128 | 37 | 63 | |
| Ceftazidime | − | 2->64 | >64 | >64 | 6 | 94 |
| + | 2->64 | 32 | >64 | 33 | 53 | |
| Aztreonam | − | 0.25->128 | 32 | 128 | 9 | 63 |
| + | 0.5->128 | 32 | 128 | 8 | 64 | |
| Imipenem | − | 0.5->64 | >64 | >64 | 8 | 88 |
| + | 0.5->64 | 16 | >64 | 17 | 75 | |
| Meropenem | − | 0.5->64 | >64 | >64 | 6 | 86 |
| + | 0.25->64 | 16 | >64 | 14 | 72 | |
| Biapenem | − | 0.12->64 | >64 | >64 | 7 | 86 |
| + | 0.12->64 | 8 | >64 | 22 | 40 | |
| Doripenem | − | 0.25->64 | >64 | >64 | 8 | 80 |
| + | 0.25->64 | 8 | >64 | 20 | 50 | |
| Non-MBL-producing P. aeruginosa (16) | ||||||
| Piperacillin | − | 4->128 | >128 | >128 | 19 | 81 |
| + | 4->128 | >128 | >128 | 25 | 75 | |
| Ceftazidime | − | 2->64 | 16 | 64 | 25 | 50 |
| + | 2->64 | 16 | 64 | 25 | 44 | |
| Aztreonam | − | 4->128 | 32 | >128 | 13 | 56 |
| + | 4->128 | 32 | >128 | 13 | 56 | |
| Imipenem | − | 1-32 | 16 | 32 | 13 | 88 |
| + | 1-32 | 16 | 32 | 13 | 81 | |
| Meropenem | − | 0.12-64 | 16 | 64 | 25 | 56 |
| + | 0.25-64 | 16 | 64 | 19 | 56 | |
| Biapenem | − | 0.5-32 | 16 | 32 | 13 | 69 |
| + | 0.5-32 | 16 | 32 | 13 | 63 | |
| Doripenem | − | 0.25-32 | 8 | 32 | 31 | 44 |
| + | 0.25-32 | 8 | 32 | 38 | 44 | |
| IMP-1-producing P. aeruginosa (166) | ||||||
| Piperacillin | − | 0.5->128 | >128 | >128 | 34 | 66 |
| + | 1->128 | 128 | >128 | 37 | 63 | |
| Ceftazidime | − | 2->64 | >64 | >64 | 7 | 93 |
| + | 2->64 | 32 | >64 | 33 | 52 | |
| Aztreonam | − | 0.25->128 | 32 | 128 | 7 | 64 |
| + | 0.5->128 | 32 | 128 | 7 | 64 | |
| Imipenem | − | 0.5->64 | >64 | >64 | 8 | 87 |
| + | 0.5->64 | 16 | >64 | 17 | 75 | |
| Meropenem | − | 0.5->64 | >64 | >64 | 6 | 86 |
| + | 0.25->64 | 16 | >64 | 13 | 72 | |
| Biapenem | − | 0.12->64 | >64 | >64 | 8 | 86 |
| + | 0.12->64 | 8 | 32 | 22 | 38 | |
| Doripenem | − | 0.25->64 | >64 | >64 | 8 | 80 |
| + | 0.25->64 | 8 | >64 | 20 | 49 | |
| VIM-2-producing P. aeruginosa (8) | ||||||
| Piperacillin | − | 64->128 | 128 | >128 | 38 | 63 |
| + | 16->128 | 128 | >128 | 38 | 63 | |
| Ceftazidime | − | 32->64 | 64 | >64 | 0 | 100 |
| + | 4->64 | 32 | >64 | 38 | 63 | |
| Aztreonam | − | 8-128 | 16 | 128 | 38 | 50 |
| + | 8->128 | 32 | >128 | 38 | 63 | |
| Imipenem | − | 16->64 | >64 | >64 | 0 | 100 |
| + | 2->64 | 64 | >64 | 13 | 75 | |
| Meropenem | − | 16->64 | >64 | >64 | 0 | 100 |
| + | 1->64 | 32 | >64 | 25 | 75 | |
| Biapenem | − | 8->64 | 64 | >64 | 0 | 75 |
| + | 0.25->64 | 16 | >64 | 25 | 75 | |
| Doripenem | − | 16->64 | 64 | >64 | 0 | 100 |
| + | 1->64 | 16 | >64 | 25 | 63 | |
Clinical and Laboratory Standards Institute (CLSI) (2010) breakpoints, where available (1), were used. CLSI (2010) does not have criteria (susceptible, intermediate, or resistant) for biapenem or doripenem (1). For comparison only, the same values for imipenem (CLSI, 2010) were used as criteria for biapenem and doripenem. S, susceptible; R, resistant.
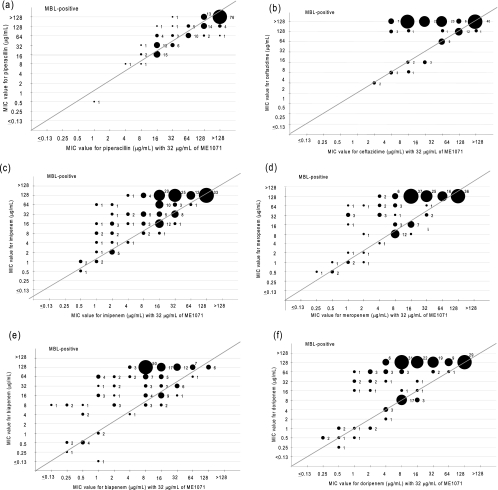
Morinaka et al. reported that a concentration of ME1071 suitable for testing its inhibitory activity with biapenem was 32 μg/ml (14). This concentration was therefore used in this study. The susceptibilities of MBL-producing and non-MBL-producing P. aeruginosa isolates to β-lactam antibiotics in the presence or absence of 32 μg/ml of ME1071 are shown in Table 1. ME1071 shows potentiation with tested carbapenems such as imipenem, meropenem, biapenem, and doripenem. Especially, the biapenem resistance rate of MBL producers in the presence of 32 μg/ml of ME1071 was remarkably decreased (40%) in comparison with biapenem alone (86%), and the susceptibility rate of MBL producers for biapenem in the presence of ME1071 (22%) was increased in comparison with biapenem alone (7%) (Table 1). ME1071 did not show potentiation with piperacillin and aztreonam against MBL-producing P. aeruginosa isolates (Fig. 2 and 3).
FIG. 2.
MIC values of 174 metallo-β-lactamase-producing Pseudomonas aeruginosa isolates against piperacillin (a), ceftazidime (b), imipenem (c), meropenem (d), biapenem (e), and doripenem (f) in the presence (x axis) or absence (y axis) of ME1071. Plot sizes and numbers in the graph represent the number of strains.
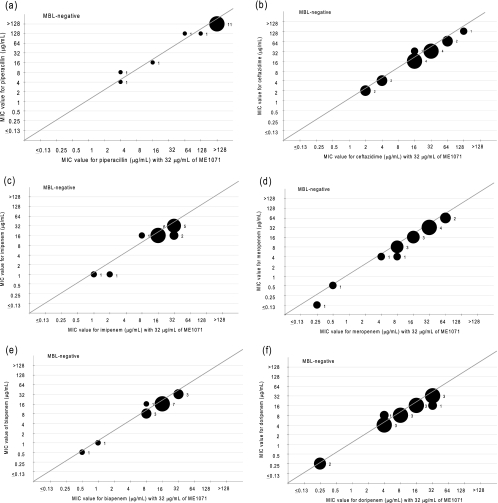
FIG. 3.
MIC values of 16 non-metallo-β-lactamase-producing Pseudomonas aeruginosa isolates against piperacillin (a), ceftazidime (b), imipenem (c), meropenem (d), biapenem (e), and doripenem (f) in the presence (x axis) or absence (y axis) of ME1071. Plot sizes and numbers in the graph represent the number of strains.
Present results, therefore, indicate that ME1071 can potentiate the activity of carbapenems and also of ceftazidime against MBL-producing strains of P. aeruginosa. MBL-producing P. aeruginosa strains are usually multidrug resistant, and MBL-producing organisms include not only P. aeruginosa but also Enterobacteriaceae, including Serratia marcescens and Proteeae (18). The only remaining antibiotics useful for treatment of infections caused by similar multidrug-resistant organisms are colistin, polymyxin B, and tigecycline (13, 16, 22). S. marcescens and Proteeae show natural resistance against polymyxins and tigecycline (22); on the other hand, P. aeruginosa is naturally resistant to tigecycline (13, 16). Therefore, the development of an MBL inhibitor may be useful for treatment of infections caused by similar multidrug-resistant organisms.
Various compounds have been reported as MBL inhibitors such as thioester derivatives, trifluoromethyl alcohols, thiols, sulfonyl hydrazones, tricyclic products, biphenyl tetrazoles, cysteinyl peptide, 1-β-methyl-carbapenem, penicillin derivatives, thioxocephalosporins, and phthalic acid derivatives (6, 17, 21). Unfortunately, there has been no MBL inhibitor suitable for clinical use until now. Osaki et al. reported a protective effect of biapenem with ME1071 by using a mouse systemic infection model (15). That report also suggested that ME1071 has low toxicity for animals compared with other MBL inhibitors.
Kinetic studies.
The purified IMP-1 and VIM-2 gave a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, and the purity of the enzyme was estimated to be >95% (data not shown). Ki values of IMP-1 and VIM-2 for ME1071 were 0.41 μM and 120 μM, respectively (Table 2). Km values of IMP-1 for carbapenems were ≥25.0 μM. On the other hand, Km values of the VIM-2 β-lactamase for carbapenems were smaller than the Ki value of VIM-2 for ME1071; however, ME1071 still shows a combinative effect with carbapenems for VIM-2 producers. The reason for these phenomena is unclear, and investigations are under way. Laraki et al. (12) and Docquier et al. (4) reported that IMP-1, VIM-1, and VIM-2 had high Km values for piperacillin compared with carbapenems or ceftazidime. This result may explain the lack of synergistic effect of ME1071 with piperacillin.
TABLE 2.
Kinetic parameters of the purified IMP-1 and VIM-2 enzymes
| Compound | IMP-1 |
VIM-2 |
||||
|---|---|---|---|---|---|---|
| kcat (s−1) | Km or Ki (μM) | kcat/Km (μM−1 s−1) | kcat (s−1) | Km or Ki (μM) | kcat/Km (μM−1 s−1) | |
| Imipenem | 63.5 ± 1.2 | 35.1 ± 3.8 | 1.8 | 98.9 ± 0.7 | 8.5 ± 0.5 | 12 |
| Meropenem | NDa | ND | 0.2 | 8.5 ± 0.3 | 9.4 ± 0.9 | 0.9 |
| Biapenem | 44.7 ± 4.7 | 167 ± 21 | 0.3 | 12.4 ± 0.3 | 24.7 ± 0.5 | 0.5 |
| ME1071b | 0.41 ± 0.1 | 120 ± 3.8 | ||||
ND, not determined.
Ki value.
Recently, the kinetic values of other MBL inhibitors have been reported (17). The Ki value of IMP-1 against J110,441 was 110 times lower than the Ki value for ME1071 (Ki, 0.41 μM); however, Ki values of IMP-1 against mercaptoacetic acid, mercaptopropionic acid, SB238569, or J110,441 were 0.18 μM to 17 μM. These data indicate that ME1071 has a high affinity for the IMP-1 enzyme compared with other current inhibitors for MBL with the exception of J110,441. Accordingly, the present data suggest that ME1071 may be a useful inhibitor for MBL-producing P. aeruginosa strains.
In summary, ME1071 is a novel MBL inhibitor derived from maleic acid that potentiates the activity of ceftazidime and carbapenems (especially biapenem) against MBL-producing P. aeruginosa. Further basic studies, including animal experiments, antibiotic susceptibility testing with other bacterial species, and detailed kinetic studies using other enzymes, are warranted in order to investigate this interesting compound.
Acknowledgments
We express our deep appreciation to Tse Hsien Koh and Kenneth S. Thomson for their critical comments and careful reviewing of the manuscript. We thank Kiyoshi Sugihara for his technical assistance.
This study was supported by a research promotion grant from Toho University Graduate School of Medicine (07-01 to Y.I.) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sport and Technology of Japan (19591185 to Y.I.).
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—8th ed., M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement, M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Cornaglia, G., M. Akova, G. Amicosante, R. Canton, R. Cauda, J. D. Docquier, M. Edelstein, J. M. Frere, M. Fuzi, M. Galleni, H. Giamarellou, M. Gniadkowski, R. Koncan, B. Libisch, F. Luzzaro, V. Miriagou, F. Navarro, P. Nordmann, L. Pagani, L. Peixe, L. Poirel, M. Souli, E. Tacconelli, A. Vatopoulos, and G. M. Rossolini. 2007. Metallo-β-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int. J. Antimicrob. Agents 29:380-388. [DOI] [PubMed] [Google Scholar]
- 4.Docquier, J. D., J. Lamotte-Brasseur, M. Galleni, G. Amicosante, J. M. Frere, and G. M. Rossolini. 2003. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 51:257-266. [DOI] [PubMed] [Google Scholar]
- 5.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J. M. Frere. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraiwa, Y., A. Morinaka, T. Fukushima, and T. Kudo. 2009. Metallo-β-lactamase inhibitory activity of phthalic acid derivatives. Bioorg. Med. Chem. Lett. 19:5162-5165. [DOI] [PubMed] [Google Scholar]
- 7.Ishii, Y., M. Galleni, L. Ma, J. M. Frere, and K. Yamaguchi. 2007. Biochemical characterisation of the CTX-M-14 β-lactamase. Int. J. Antimicrob. Agents 29:159-164. [DOI] [PubMed] [Google Scholar]
- 8.Ishii, Y., K. Tateda, and K. Yamaguchi. 2008. Evaluation of antimicrobial susceptibility for β-lactams using the Etest method against clinical isolates from 100 medical centers in Japan (2006). Diagn. Microbiol. Infect. Dis. 60:177-183. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby, G. A. 2009. AmpC β-lactamases. Clin. Microbiol. Rev. 22:161-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura, S., J. Alba, K. Shiroto, R. Sano, Y. Niki, S. Maesaki, K. Akizawa, M. Kaku, Y. Watanuki, Y. Ishii, and K. Yamaguchi. 2005. Clonal diversity of metallo-β-lactamase-possessing Pseudomonas aeruginosa in geographically diverse regions of Japan. J. Clin. Microbiol. 43:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh, T. H., K. Yamaguchi, and Y. Ishii. 2008. Characterisation of the metallo-β-lactamase VIM-6 and its genetic support. Int. J. Antimicrob. Agents 32:446-449. [DOI] [PubMed] [Google Scholar]
- 12.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frere, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltezou, H. C. 2009. Metallo-β-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? Int. J. Antimicrob. Agents 33:405.e1-7. [DOI] [PubMed] [Google Scholar]
- 14.Morinaka, A., Y. Osaki, T. Fukushima, T. Mikuniya, T. Yoshida, and M. Yonezawa. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-330, p. 225.
- 15.Osaki, Y., A. Morinaka, T. Mikuniya, Y. Sanbongi, T. Ida, T. Yoshida, and M. Yonezawa. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-332, p. 226.
- 16.Paterson, D. L. 2008. Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin. Infect. Dis. 47(Suppl. 1):S14-S20. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Llarena, F. J., and G. Bou. 2009. β-Lactamase inhibitors: the story so far. Curr. Med. Chem. 16:3740-3765. [DOI] [PubMed] [Google Scholar]
- 18.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata, N., Y. Doi, K. Yamane, T. Yagi, H. Kurokawa, K. Shibayama, H. Kato, K. Kai, and Y. Arakawa. 2003. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 41:5407-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syrmis, M. W., M. R. O'Carroll, T. P. Sloots, C. Coulter, C. E. Wainwright, S. C. Bell, and M. D. Nissen. 2004. Rapid genotyping of Pseudomonas aeruginosa isolates harboured by adult and paediatric patients with cystic fibrosis using repetitive-element-based PCR assays. J. Med. Microbiol. 53:1089-1096. [DOI] [PubMed] [Google Scholar]
- 21.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavascki, A. P., L. Z. Goldani, J. Li, and R. L. Nation. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60:1206-1215. [DOI] [PubMed] [Google Scholar]