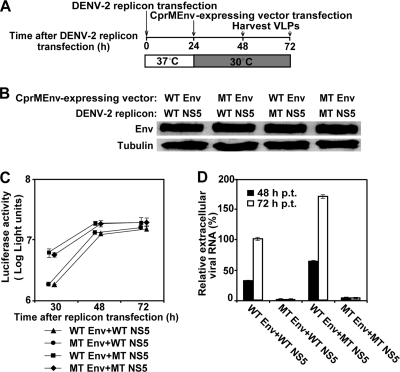
FIG. 8.
VLP analysis of NS5 and envelope protein mutations. (A) Scheme for DENV-2 VLP production. VLPs were prepared by sequential transfection of BHK-21 cells with a luciferase-reporting replicon and a vector expressing viral CprMEnv proteins. See Materials and Methods for details. (B) Western blotting of WT and mutant (MT) envelope proteins. The doubly transfected cells were lysed at 30 h after the first electroporation. The lysates were blotted for envelope proteins using mouse monoclonal 4G2 IgG and horseradish peroxidase-labeled anti-mouse IgG as primary and secondary antibodies, respectively; γ-tubulin was also blotted as a loading control. The combinations of WT and NS5 mutant replicons and WT and Env mutant structural proteins are indicated above the blotting image. (C) Kinetics of replicon replication in VLP packaging cells. Luciferase activities were measured from the doubly transfected VLP packaging cells at the indicated time points after the first replicon transfection. Average results from three experiments are shown. (D) Effects of envelope protein and NS5 mutations on VLP assembly/release. VLP production in culture fluid was measured by determination of the amount of extracellular viral RNA at 48 and 72 h after the first transfection. The relative amounts of RNA are shown; the amount of viral RNA derived from the VLP (collected at 72 h p.t.) containing the WT replicon and WT structural proteins was set equal to 100%. Average results from three independent experiments are presented.