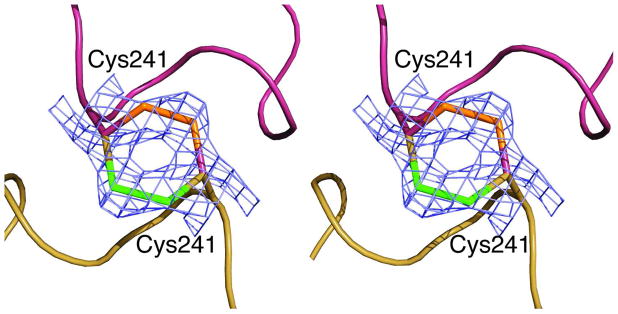
Figure 5.
Disulfide linkages between upper and lower catalytic subunits. Stereoview of the 240’s loop from a single catalytic chain from the upper catalytic trimer, maroon, and a single catalytic chain from the lower catalytic trimer, gold. These two catalytic chains are covalently linked via a disulfide bond formed between the side chains of Cys241 residues in the upper and lower catalytic chains. The refined coordinates of residue Cys241 from both chains are overlaid on the 2Fo–Fc electron density map (blue) contoured at 1.0 σ. The Cys241 residue exists in two alternate conformations, each with 50% occupancy. The sulfur atoms involved in the bonds are green for one conformation and orange for the other. One position of the disulfide bond is outlined with maroon carbons and the other position with gold carbons.