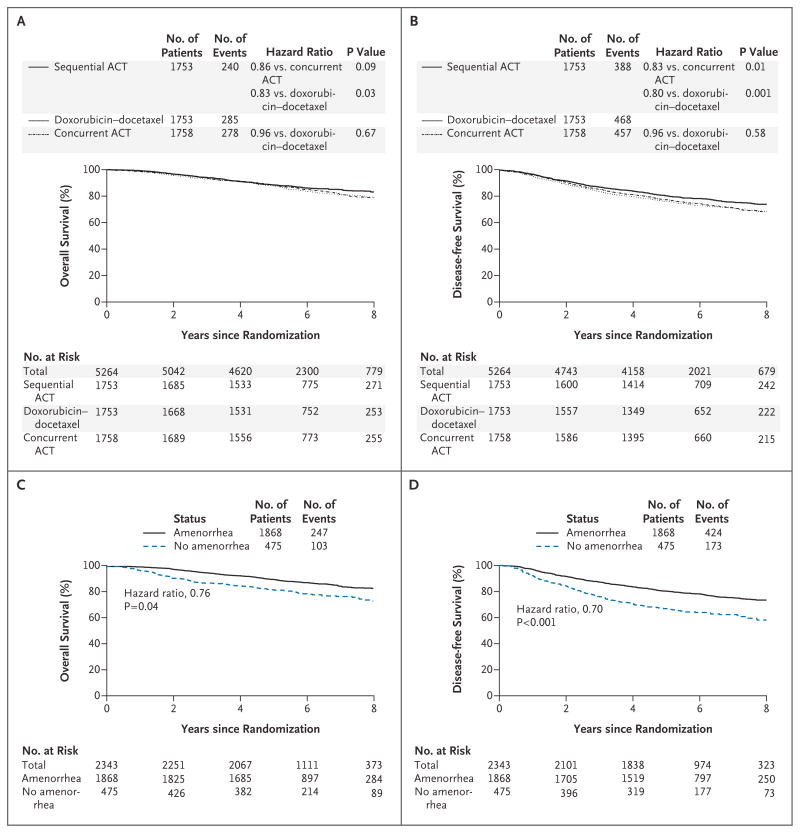
Figure 2. Overall Survival and Disease-free Survival.
Panels A and B show the Kaplan–Meier estimates of survival and disease-free survival in the three treatment groups over the follow-up period. P values are by two-sided log-rank tests. At the prespecified significance level of 0.05, a significant improvement in overall survival was associated with doxorubicin and cyclophosphamide followed by docetaxel (sequential ACT) as compared with doxorubicin–docetaxel (P = 0.03), and a marginal benefit was associated with sequential ACT over concurrent administration of doxorubicin, docetaxel, and cyclophosphamide (concurrent ACT) (P = 0.09) (Panel A). There was a statistically significant decrease in disease recurrence, a second malignant condition, or death in the sequential-ACT group as compared with the concurrent-ACT group (P = 0.01). In addition, the rate of disease-free survival was significantly higher among patients in the sequential-ACT group than among patients in the doxorubicin–docetaxel group (P = 0.001) (Panel B). Panels C and D show the effect of amenorrhea on overall survival and disease-free survival, respectively, for the combined treatment groups adjusted for treatment, estrogen-receptor status, age, lymph-node status, tumor size, and use or nonuse of hormone therapy. Overall survival (hazard ratio for death, 0.76; P = 0.04) and disease-free survival (hazard ratio for disease recurrence, a second malignant condition, or death, 0.70; P<0.001) were improved among patients with amenorrhea for 6 months or more.