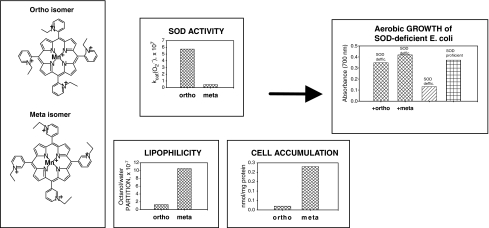
FIG. 7.
Higher lipophilicity of meta Mn(III) N-alkylpyridylporphyrins drives their higher accumulation inside E. coli and compensates for lower antioxidant potency when compared with ortho analogues. Consequently, meta and ortho isomers are similarly efficacious in protecting SOD-deficient E. coli that lacks cytosolic SOD (178). Here, the most obvious case with ortho and meta N-ethylpyridylporpyrin is illustrated: meta isomer is ∼10-fold less SOD-active than the ortho species, but is ∼10-fold more lipophilic and accumulates ∼10-fold more in E. coli. In turn, both compounds are equally efficient in substituting for cytosolic superoxide dismutases.