To the Editor
Staphylococcus aureus (S. aureus) infection is a known trigger for skin inflammation and can modulate immune responses. Atopic dermatitis (AD), a chronic inflammatory pruritic skin disease, affects 10–20% of children and 1–3% of adults (De Benedetto et al., 2009). Due to the loss of skin integrity by scratching, as well as decreased levels of antimicrobial peptides in comparison to normal skin or other inflammatory diseases such as psoriasis (Leung, 2003; Ong et al., 2002), patients with AD are particularly susceptible to staphylococcal skin infections, which can further worsen their skin disease (Bieber, 2008). Studies have suggested several underlying mechanisms for staphylococcus-mediated inflammation, which include production of inflammatory cytokines following either direct infection of keratinocytes or immune cells by the bacteria, or indirectly by bacterial products (Baker, 2006; Leung, 2003; Sasaki et al., 2003; Travers et al., 2001). We have demonstrated previously that lipoteichoic acid (LTA), a gram-positive bacterial lipoprotein, may be an important component of the ability of S. aureus to exacerbate AD lesions (Travers et al., 2010). In the present study, we report that staphylococcal protein A (SPA) could also contribute to this process.
SPA is a 40–60 kDa bacteria surface protein. It binds to the Fc region of IgG via interaction with the heavy chain, which disrupts opsonization and phagocytosis, contributing to the virulence of S. aureus (Foster, 2005). Studies have shown that SPA can also activate the tumor necrosis factor receptor-1 (TNFR1), which leads to NF-κB and AP-1 activation and subsequent production of cytokines and chemokines, promoting the inflammatory response in staphylococcal pneumonia (Gomez et al., 2004). Though SPA has potent biological effects, a potential pathologic role for this staphylococcal protein, or the amounts found within infected skin lesions remains largely unknown.
To assess SPA levels in infected AD lesions, we enrolled a total of 89 children (ages 3 months to 6 years) with clinically impetiginized AD diagnosed using criteria of Hanifen and Rajka (Travers et al., 2010). 63 patients returned for their second visit following a 2-week regimen of oral antibiotics (cephalexin or clindamycin if allergic to cephalosporins or penicillins) and topical corticosteroids. The experimental procedures were previously described (Travers et al., 2010). At each visit, subjects underwent a clinical assessment of a clinically-infected lesion of dermatitis using the eczema area and severity index (EASI) score (Hanifin et al., 2001). Lesional wash fluid was collected, aliquotted and stored at −80 °C until use. S. aureus, S. epidermidis (coagulase-negative staphylococcus) and group A streptococcus colonies were quantified by limiting dilution assay. Quantitative measurement of SPA was performed using ELISA (Assay Designs Inc., Ann Arbor, MI). Measurements of LTA and cytokines interleukin (IL)-6, IL-8, and TNF-α were as previously described (Travers et al., 2010). The levels of SPA, LTA and cytokines in wash fluid were first calculated based upon area of the chamber (ng/cm2), and then converted to volume (ng/cm3) based upon estimation of 0.1 cm effective epidermal thickness.
S. aureus was detected from clinically impetiginized AD lesions in 88.8% (79/89) subjects at their first visit. Treatment with antibiotics resulted in decreased amounts of S. aureus on the lesional skin samples and improvement of the lesional EASI scores (Travers et al., 2010).
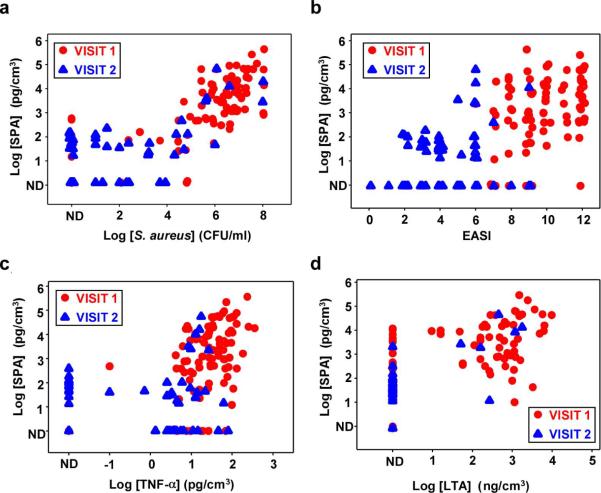
Measurable amounts of SPA were found in 91.0% (81/89) first visit and 55.6% (35/63) second visit samples. Of note, 64.0% (57/89) first visit samples but only 9.5% (6/63) second visit samples had SPA levels > 0.5 ng/cm3. As depicted in Figure 1a, there was a strong positive correlation between amounts of SPA and staphylococcal bacterial CFU (visit 1: r = 0.71, p < 0.0001; visit 2: r = 0.45, p = 0.0002). In addition, the levels of SPA was positively correlated with the lesional EASI score at visit 1 (visit 1: r = 0.36, p = 0.0005; visit 2: r = 0.15, p = 0.23) (Figure 1b).
Figure 1. Correlations between amounts of SPA and amounts of S. aureus, levels of TNF-α and LTA in lesional wash fluids, and clinical assessment of inflammation in AD patients.
Wash fluids were obtained from 89 first-visit (red circle) and 63 second-visit (blue triangle) subjects. (a) Amounts of SPA correlate with S. aureus bacteria. Spearman Correlation coefficient visit 1 r = 0.71; p < 0.0001; visit 2 r = 0.45; p = 0.0002. (b) Amounts of SPA correlate with lesional EASI scores. Spearman Correlation coefficient visit 1 r = 0.36; p = 0.0005; visit 2 r = 0.15; p = 0.23. (c) Amounts of SPA correlate with levels of TNF-α. Spearman Correlation coefficient visit 1 r = 0.41; p < 0.0001; visit 2 r = 0.04; p = 0.78. (d) Amounts of SPA correlate with levels of LTA. Spearman Correlation coefficient visit 1 r = 0.55; p < 0.0001.
S. aureus infection or SPA itself has been shown to induce the pro-inflammatory cytokine TNF-α in primary human keratinocytes (Aufiero et al., 2007; Ezepchuk et al., 1996). Our previous studies indicated that biologically relevant levels of pro-inflammatory cytokines can be measured in many wash fluid specimens derived from clinically impetiginized AD lesions (Travers et al., 2010). As shown in Figure 1c, the levels of SPA was positively correlated with the amounts of TNF-α for the first visit samples (r = 0.41, p < 0.0001). Moreover, there was a positive correlation between SPA and other pro-inflammatory cytokines including IL-6 and IL-8 (data not shown).
The dose of SPA needed to induce cutaneous reactions in human skin has been reported to be in the ng/ml range (White and Noble, 1985). Therefore, pharmacologically active (i.e, above 10 ng/cm3) levels of SPA are commonly encountered (30% of first visits) in subjects with impetiginized atopic dermatitis lesions. These data suggested that SPA might contribute to the worsening of AD lesions by S. aureus infection. In fact, it has been shown that topical application of SPA after destroying skin barrier function with detergent can induce AD-like skin inflammation in mice (Terada et al., 2006). However, it is also possible that the increased skin inflammation might be simply caused by other coexisting bacterial products such as LTA and staphylococcal toxins that have been implicated in the worsening of AD in response to infection (Ezepchuk et al., 1996; Travers et al., 2010; Travers et al., 2001). Indeed, we observed a strong positive correlation between SPA and LTA among first visit samples (r = 0.55, p < 0.0001) (Figure 1d). It also remains unclear at this point how SPA induces skin inflammation, e.g. the cell type(s) and receptor(s) being involved. Further investigations are warranted to elucidate the role of SPA in the pathogenesis of S. aureus-mediated worsening of AD.
Acknowledgements
This research was supported in part by grants from the Riley Memorial Association, and the National Institutes of Health grants HL62996 (JBT), U19 AI070448 (MK, JBT) and Veteran's Administration Merit Award (JBT).
Abbreviations
- AD
Atopic dermatitis
- CFU
colony forming unit
- EASI
eczema area and severity index
- IL
interleukin
- LTA
Lipoteichoic acid
- S. aureus
Staphylococcus aureus
- SPA
Staphylococcal protein A
- TNF
Tumor necrosis factor
Footnotes
Conflict of Interest The authors state no conflict of interest.
References
- Aufiero B, Guo M, Young C, Duanmu Z, Talwar H, Lee HK, et al. Staphylococcus aureus induces the expression of tumor necrosis factor-alpha in primary human keratinocytes. International journal of dermatology. 2007;46:687–694. doi: 10.1111/j.1365-4632.2007.03161.x. [DOI] [PubMed] [Google Scholar]
- Baker BS. The role of microorganisms in atopic dermatitis. Clinical and experimental immunology. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. The New England journal of medicine. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA. Atopic dermatitis: a disease caused by innate immune defects? The Journal of investigative dermatology. 2009;129:14–30. doi: 10.1038/jid.2008.259. [DOI] [PubMed] [Google Scholar]
- Ezepchuk YV, Leung DY, Middleton MH, Bina P, Reiser R, Norris DA. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. The Journal of investigative dermatology. 1996;107:603–609. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by staphylococci. Nature reviews. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nature medicine. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Experimental dermatology. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- Leung DY. Infection in atopic dermatitis. Current opinion in pediatrics. 2003;15:399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England journal of medicine. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kano R, Sato H, Nakamura Y, Watanabe S, Hasegawa A. Effects of staphylococci on cytokine production from human keratinocytes. The British journal of dermatology. 2003;148:46–50. doi: 10.1046/j.1365-2133.2003.05017.x. [DOI] [PubMed] [Google Scholar]
- Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Kozman A, Mousdicas N, Saha C, Landis M, Al-Hassani M, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. The Journal of allergy and clinical immunology. 2010;125:146–152. e141–142. doi: 10.1016/j.jaci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norris DA, Leung DY. The keratinocyte as a target for staphylococcal bacterial toxins. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc. 2001;6:225–230. doi: 10.1046/j.0022-202x.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- White MI, Noble WC. The cutaneous reaction to staphylococcal protein A in normal subjects and patients with atopic dermatitis or psoriasis. The British journal of dermatology. 1985;113:179–183. doi: 10.1111/j.1365-2133.1985.tb02062.x. [DOI] [PubMed] [Google Scholar]