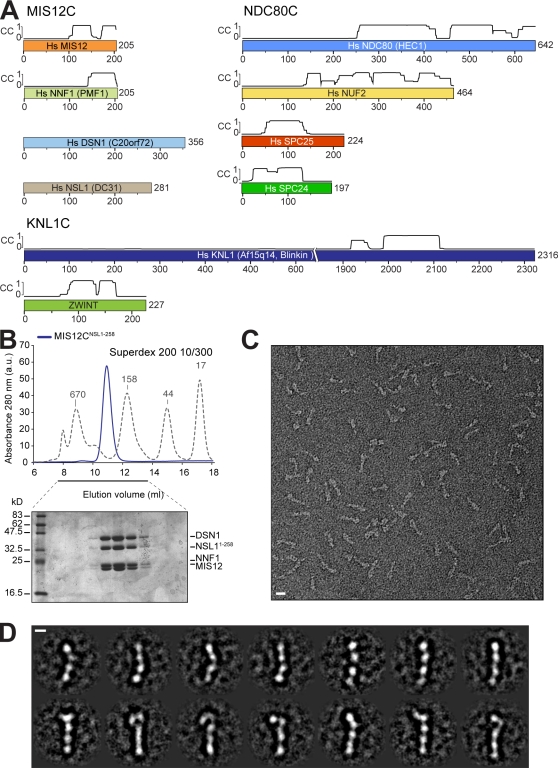
Figure 1.
Reconstitution and structural analysis of human MIS12C. (A) Schematic representation of the components of the MIS12C, NDC80C, and KNL1C. Coiled-coil (CC) predictions calculated with program COILS (Lupas et al., 1991) are shown exclusively for subunits with partial or complete coiled-coil content. Alternate names in humans are indicated. Hs, Homo sapiens. (B) Size-exclusion chromatography run of recombinant MIS12CNSL1-258 with corresponding SDS-PAGE separation stained with Coomassie brilliant blue. The molecular mass of the recombinant complex is ∼120 kD, but the protein elutes earlier than expected for a globular protein of equivalent molecular mass, suggesting that it is an oligomer or that it is elongated. The dashed gray line and numbers indicate elution markers in the size-exclusion chromatography experiments and their molecular masses (in kilodaltons), respectively. a.u., arbitrary unit. (C) Negative-stain EM was performed on the recombinant MIS12CNSL1-258. The maximum length of the complex varies between ∼21 and 23 nm depending on the curvature. The maximum thickness of the rodlike structures is ∼3 nm. (D) The class averages represent the characteristic views of the MIS12C and reveal a varying amount of curvature. Bars: (C) 10 nm; (D) 5 nm.