Figure 4.
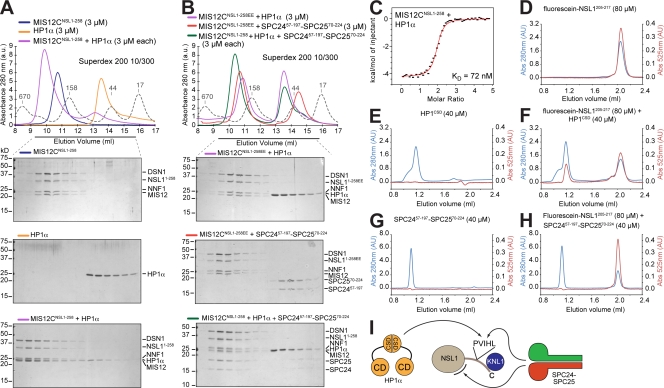
Interaction of MIS12C with HP1. (A) Size-exclusion chromatography elution profiles and SDS-PAGE analysis of MIS12CNSL1-258 (top), HP1-α (middle), and their stoichiometric combination (bottom). (B) The MIS12CNSL1-258EE mutant does not bind HP1-α (top). SPC2457–197–SPC2570–224 is also unable to bind MIS12CNSL1-258EE, indicating that the binding sites for HP1-α and SPC2457–197–SPC2570–224 overlap, at least in part (middle). When HP1-α and SPC2457–197–SPC2570–224 were combined in stoichiometric amounts with MIS12CNSL1-258, HP1-α did not coelute with MIS12CNSL1-258, whereas SPC2457–197–SPC2570–224 was incorporated in a complex with MIS12CNSL1-258, suggesting that SPC2457–197–SPC2570–224 binds MIS12CNSL1-258 with higher affinity (bottom). (A and B) Dashed gray lines and numbers indicate elution markers in the size-exclusion chromatography experiments and their molecular masses (in kilodaltons), respectively. (C) ITC binding curve for the interaction of MIS12CNSL1-258 with HP1-α. (D) Elution profile from a size-exclusion chromatography Superdex 75 PC 3.2/30 column of a fluorescein-labeled synthetic peptide encompassing residues 205–217 of NSL1 (fluorescein-NSL1205–217). The red trace reports absorbance (Abs) at 525 nm. Panels D through H were run under the same experimental conditions. (E) Elution profile of the chromoshadow domain of HP1-α. (F) Elution profile of a mixture of the chromoshadow domain of HP1-α and fluorescein-NSL1205–217 demonstrating a shift in the elution profile of the fluorescein-NSL1205–217 peptide. (G) Elution profile of SPC2457–197–SPC2570–224. (H) The SPC2457–197–SPC2570–224 construct does not coelute with the fluorescein-NSL1205–217 peptide, suggesting that this region of NSL1 is insufficient for high-affinity binding to NDC80C. (I) Summary of interactions presented in the figure. The position of the second binding site for SPC24–SPC24, indicated by a black curved segment, is actually on MIS12C, but not necessarily on the NSL1 subunit as shown. CD, chromodomain; CSD, chromoshadow domain.