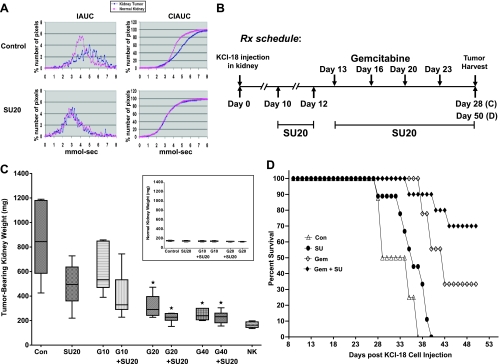
Figure 1.
KCI-18 kidney tumor response to sunitinib combined with gemcitabine. (A) DCE-MRI of early vascular changes induced by sunitinib. Mice bearing established kidney tumors were treated daily with sunitinib at 20 mg/kg per day (SU20) for 3 days and imaged by DCE-MRI. (B) Treatment schedule for combination therapy. Mice bearing established kidney tumors were pretreated with sunitinib at 20 mg/kg per day (SU20) for 3 days on days 10 to 12 after KCI-18 cell injection in the kidney. Then, mice received gemcitabine treatments at 10, 20, or 40 mg/kg given 3 days apart, twice a week for 2 weeks on days 13, 16, 20, and 23. Sunitinib was continued daily for up to 28 days for a short-term experiment (C) or for 50 days for a longer-term experiment (D). (C) Response of tumor-bearing kidneys to single and combined therapy. On day 28, tumor-bearing kidneys and contralateral normal kidneys were resected and weighed. The weights of the tumor-bearing kidneys and their median are reported for six to eight mice per group treated with vehicle (control) or sunitinib at 20 mg/kg per day (SU20) or gemcitabine at 10 (G10), 20 (G20), or 40 mg/kg (G40); each drug alone and in combination compared with the normal contralateral kidney weights (NK). Inset shows weights of the normal contralateral kidneys for each treatment group. *P < .001. (D) Survival of KCI-18 kidney tumor-bearing mice treated with sunitinib combined with gemcitabine. Mice bearing established kidney tumors were pretreated with sunitinib (SU) at 20 mg/kg per day for 3 days on days 10 to 12 after KCI-18 cell injection in the kidney. Then, mice received five gemcitabine (Gem) treatments at 20 mg/kg given 3 to 4 days apart, during 3 weeks on days 13, 16, 20, 23, and 27, and sunitinib was continued daily, 5 days per week, for up to 50 days as shown in Figure 2B. Mice were followed for survival and Kaplan-Meier survival curves of mice treated with vehicle (Con for control) sunitinib (SU) or gemcitabine (Gem) or both combined (Gem + SU) were constructed.