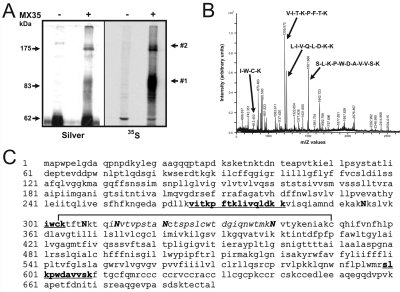
Figure 1.
Mass spectrometry sequencing by fragmentation of peptides. Cell surface expressed MX35 antigen was isolated from two different MX35 antigen-positive cell lines, OVCAR-3 and SK-RC-18, by immunoprecipitation following metabolic labeling of proteins with 35S methionine and 35S cysteine. (A) SDS-PAGE of MX35 immune complexes labeled with 35S and visualized by autoradiofluorography or by silver staining. Monoclonal antibody MX35 precipitated the antigen in two forms, band #1 and #2, differing in size. (B) Peptide mass fingerprinting of trypsin digested Lys-tagged and sulfonated protein. (C) Sequence of the sodium-dependent phosphate transporter 2b protein. Peptides detected by mass spectrometry are shown in bold. The putative disulfide-bonded loop (aa 303-350) is also shown. The region containing the epitope recognized by mAb MX35 is shown in italics. Asparagine (N) residues that are probable N-linked glycosylation sites are shown in capital letters.