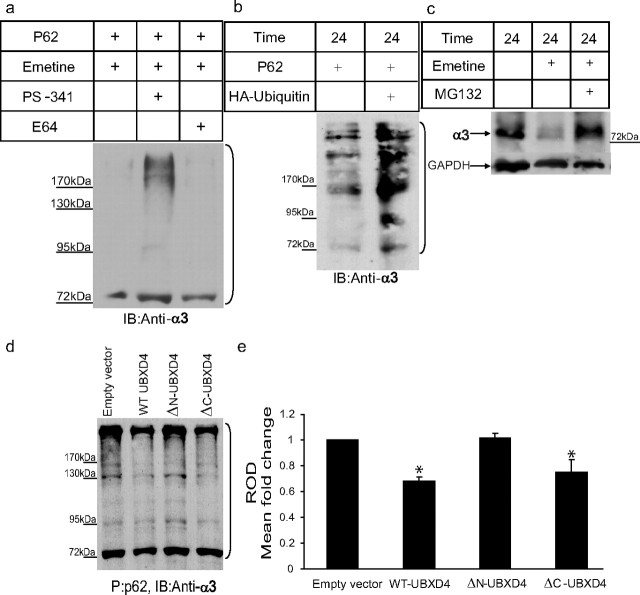
Figure 7.
UBXD4 decreases the levels of ubiquitinated α3. Differentiated PC12 cells were treated for 24 h with PS-341 (a selective proteasome inhibitor) or E64 (an inhibitor of the lysosome) in the presence of emetine (a protein synthesis blocker). Cell lysates were probed for α3. a, A ladder of various polyubiquitinated forms of α3 (bracket) could be pulled down by the p62-derived UBA domain, suggesting that α3 is ubiquitinated and can be degraded by the proteasome. In untreated cells (left lane) and cells treated with E64 (right lane), the majority of α3 receptors was degraded by the proteasome after ubiquitination. b, In another set of experiments, the level of ubiquitination of α3 in the presence of overexpressed HA-ubiquitin was determined. Cell lysates of PC12 cells stably expressing empty vector or HA-ubiquitin were subjected to p62 pull down followed by immunoblotting with anti-α3 antibodies. Overexpression of HA-ubiquitin led to an increase in the levels of ubiquitinated α3. c, The degradation of the α3 nAChR subunit was decreased dramatically in the presence of the proteasome inhibitor MG132 in PC12 cells. d, P62 pull down was performed on cell lysates from dPC12 cells stably transfected with the corresponding empty vector, WT-UBXD4, ΔN-UBXD4, or ΔC-UBXD4 followed by Western blot analysis with anti-α3 antibodies. A ladder of ubiquitinated α3 could be detected in each sample. Quantification of the α3 signal showed that WT-UBXD4 and ΔC-UBXD4, but not ΔN-UBXD4, significantly decrease the ubiquitinated levels of α3 (e; p < 0.05). The brackets on the right (a, b, d) mark a ladder of bands corresponding to polyubiquitinated α3.