Table 2.
Ag2O-catalyzed fluorination of complex small molecules.
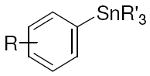 R′ = Me, Bu |
 |
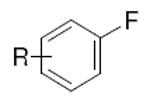 |
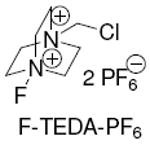 F-TEDA-PF6 |
|
|---|---|---|---|---|
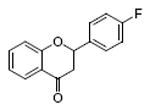 flavanone 90%, 8 |
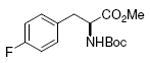 Boc-Tyr-OMe 85%, 9 |
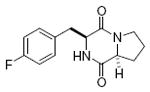 maculosin 78%, 10 |
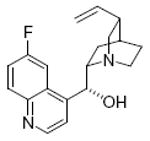 quinine 75%, 11a |
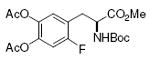 DOPA 78%, 12 |
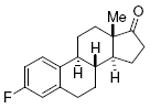 estrone 81%, 13 |
Leu-enkephalin 83%, 14 |
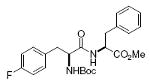 Boc-Tyr-Phe-OMe 92%, 15 |
||
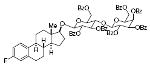 estrol-17-β-D-lactosde heptabenzoate 80%, 16 |
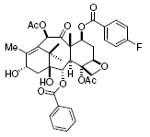 taxol 72%, 17 |
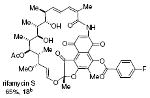 rifamycin S 65%, 18b |
||
20 mol% of AgOTf, 2 equiv of NaOTf and 2 equiv of F-TEDA-PF6 were used at 90 °C.
20 mol% of AgOTf, 2 equiv of NaOTf, and 5 equiv of MeOH was used. The names under the molecules in Table 2 refer to the parent molecules and not to fluorinated analogs shown.
