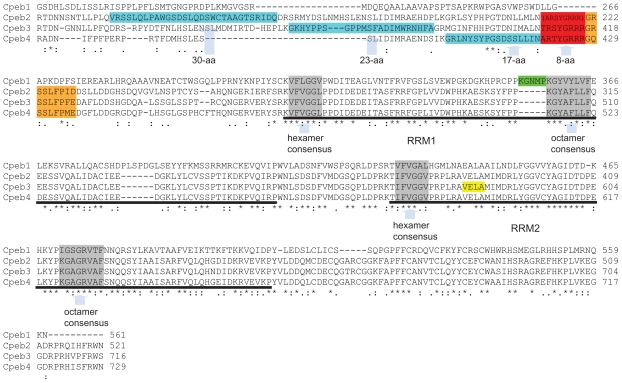
Figure 5.
Comparison of the conserved regions in mouse Cpeb1–4 protein. The alternatively spliced 17~30-aa regions are highlighted in blue, the alternatively spliced 8-aa in red, and the alternatively spliced 9-aa in orange. The parentheses indicate that all Cpeb2 isoforms recorded in the UniProt database have the 8-aa deleted. Cpeb2, 3, and 4 share high homology in the 8-aa and the 9-aa regions, but little homology in the 17~30-aa region. The underlined sequences represent the first and second RRMs, respectively, in all four CPEBs. The regions in grey are hexamer and octamer consensus sequences within the RRMs. The hexamer and octamer consensus sequences within the RRMs and the linker between two RRMs are identical in Cpeb2–4, suggesting that it is highly likely that Cpeb2–4 share the same protein/RNA interaction mechanisms. The sequences surrounding the consensuses, N terminal to the first RRM, and C terminal to the second RRM are similar among Cpeb2–4, with a few amino acid replacements. This suggests that Cpeb2–4 recognizes similar substrates. In contrast, Cpeb1 demonstrates significant differences to Cpeb2–4 within these regions, including the consensus sequences, suggesting that Cpeb1 not only employs a distinct mechanism for protein/RNA interaction, but also targets a different group of RNAs. The insertion of the 5-aa in Cpeb1 (highlighted in green) is adjacent to the octamer consensus in the first RRM, possibly posing a potential impact on its specificity. An early termination with an altered tail which alters VELA (highlighted in yellow) to GEWK disrupts the second RRM in a Cpeb3 isoform. This may pose an impact on both the binding mechanism and the substrate specificity of Cpeb3. Accession numbers used for the alignment are as follows: Cpeb1: NP_031781, Cpeb2: NP_787951; Cpeb3: NP_938042; Cpeb4: NP_080528. Alignment was achieved with the aid of ClustalW2. Asterisks represent perfect matches; colons represent substitutions with similar amino acids; periods represent substitutions with rather distinct amino acids.