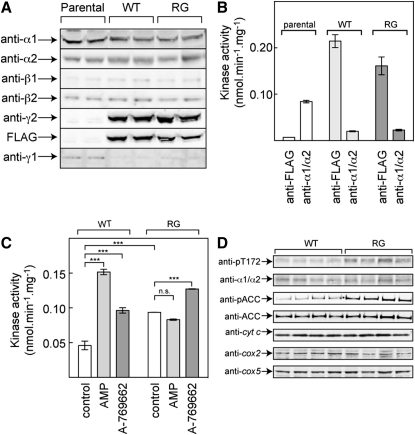
Figure 1.
Analysis of HEK293 Cells Stably Expressing WT γ2 and the R531G Mutant
(A) Duplicate western blots of parental HEK293 cells and WT or RG cells using antibodies against the α1, α2, β1, β2, γ1, and γ2 subunits of AMPK, and against the FLAG epitope on γ2.
(B) AMPK activities in parental HEK293 cells and WT and RG cells, in samples made by immunoprecipitation using anti-FLAG antibodies, and by subsequent immunoprecipitation from the anti-FLAG supernatants using anti-α1/α2 antibodies. Results (mean ± SD, n = 2) are expressed as units per milligram of protein in the volume of cell lysate from which the first immunoprecipitation was performed.
(C) Effect of AMP (200 μM) and A769662 (1 μM) in cell-free assays of anti-FLAG immunoprecipitates from WT and RG cells. Results expressed as in (B). Significant differences between indicated groups were determined by one-way ANOVA (∗∗∗p < 0.001; n.s., not significant).
(D) Western blots (n = 4) showing phosphorylation (pT172) and expression (α1/α2) of AMPK, phosphorylation (pACC) and expression (ACC) of acetyl-CoA carboxylase, and expression of three mitochondrial markers (cyt c, cytochrome c; cox2, flavoprotein subunit of succinate dehydrogenase [SDHA]; cox5, ATP synthase, F1 complex, β subunit) in WT and RG cells.