Abstract
Background and purpose:
Growing evidence implicates NF-κB as an important contributor to metastasis and increased chemoresistance of melanoma. Here, we report the effects of parthenolide on either untreated, cisplatin- or TNFα-treated melanoma cell lines A375, 1205Lu and WM793, exhibiting different levels of constitutive NF-κB activity.
Experimental approach:
Electrophoretic mobility shift assay was used to assess changes in NF-κB activity, and real-time PCR to evaluate expression of NF-κB-regulated genes. Cell cycle arrest and apoptosis were assessed by flow cytometry. Cell death was also visualized by fluorescence microscopy. Migration was determined by scratch assay and invasiveness by Matrigel assay.
Key results:
Parthenolide suppressed both constitutive and induced NF-κB activity in melanoma cells. This was accompanied by down-regulation of cancer-related genes, with NF-κB-binding sites in their promoters, including: Bcl-XL, survivin, cyclin D1, interleukin 8 and matrix metalloproteinase 9. When the various effects of 6 µM parthenolide were compared, apoptosis associated with loss of mitochondrial membrane potential was most efficiently induced in 1205Lu cells, cell cycle arrest in G0/G1 phase was observed in WM793 cells, and high metastatic potential was markedly reduced in A375 cells. These findings not only reflected differences between melanoma cell lines in basal expression of NF-κB-regulated genes, but also suggested other parthenolide targets involved in cell cycle progression, migration, invasiveness and survival.
Conclusions:
Inhibition of constitutive and therapeutically induced NF-κB pathway by parthenolide might be useful in the treatment of melanoma, although the diversity of changes induced in melanoma cells with different genetic backgrounds indicate context-dependent poly-pharmacological properties of this compound.
Keywords: apoptosis, cell cycle arrest, cisplatin, invasiveness, melanoma, NF-κB pathway, parthenolide, polypharmacology
Introduction
Cutaneous melanoma, the most aggressive form of skin cancer, is highly resistant to chemo-, radio- and immunotherapy, and long-term survival of patients with metastatic melanoma is rare. Novel therapy strategies for melanoma are therefore urgently needed, especially when the increase in incidence observed lately is considered (Jemal et al., 2008).
The sesquiterpene lactone parthenolide is an active component of the herb feverfew (Tanacetum parthenium), which has been used for a long time for the treatment of inflammation, migraine, menstrual irregularities, fever and rheumatoid arthritis. Recent studies have revealed that parthenolide also has anti-proliferative and pro-apoptotic effects against a variety of tumours both in vitro and in animal models (see Zhang et al., 2005; Bedoya et al., 2008). Particularly, parthenolide combined with arsenic trioxide (Duechler et al., 2008) or lactacystin (Cory and Cory, 2002) in leukaemia cells, and with sulindac in pancreatic carcinoma (Yip-Schneider et al., 2005), mediated growth suppression and apoptosis and it reduced metastasis in combination with docetaxel (Sweeney et al., 2005). Moreover, parthenolide was one of the first reported drugs showing toxicity against cancer stem cells, either alone (Guzman et al., 2005; Zhou et al., 2008; Kawasaki et al., 2009) or in combination with vinorelbine (Liu et al., 2008). The mechanisms underlying the anti-cancer properties of parthenolide are diverse. A structure-function relationship study has indicated that parthenolide is able to interact with nucleophilic sites of proteins such as exposed cysteine residues (Hehner et al., 1998). It can promote apoptosis of cancer cells through increasing intracellular concentration of reactive oxygen species (ROS) (Wen et al., 2002; Wang et al., 2006), and sustained activation of c-Jun N-terminal kinase (Nakshatri et al., 2004). Recently, it has been demonstrated that parthenolide can deplete histone deacetylase 1 (HDAC1) (Gopal et al., 2007), inhibit tubulin carboxypeptidase activity (Fonrose et al., 2007) and promote the ubiquitination of murine double minute 2 (MDM2) (Gopal et al., 2009). Other studies have revealed that parthenolide promotes in vitro and in vivo DNA hypomethylation by inhibiting DNA methyltransferase 1 (DNMT1) (Liu et al., 2009). The inhibition of the transcription factor NF-κB by parthenolide via targeting the IKK (IkappaB kinase) complex was first observed in HeLa cells (Bork et al., 1997). Parthenolide can also suppress NF-κB DNA-binding activity via direct modification of the p65 subunit (García-Piñeres et al., 2001). Active NF-κB modulates the expression of a number of genes involved in cell survival, proliferation, angiogenesis, metastasis and inhibition of apoptosis (Baud and Karin, 2009). NF-κB has been reported to be constitutively activated in many cancers including melanoma (Melnikova and Bar-Eli, 2008; Baud and Karin, 2009; Yang et al., 2009). Therefore, parthenolide as an inhibitor of IKK activity, and/or DNA-binding activity of p65 subunit of NF-κB, could be considered to have promise in the treatment of melanoma. Moreover, inhibition of NF-κB might sensitize cancer cells to death-inducing stimuli including chemotherapeutic drugs. Increased efficacy of anti-cancer treatment by combining the inhibition of NF-κB pathway with conventional chemotherapy has already been shown for ovarian cancer (Mabuchi et al., 2004), breast cancer (Patel et al., 2000) and melanoma (Schön et al., 2008).
In a previous study, preincubation of parthenolide with the thiol nucleophile N-acetyl-cysteine protected melanoma cells from parthenolide-induced cell death suggesting reaction with intracellular thiols as the mechanism responsible for parthenolide activity in melanoma cells (Lesiak et al., 2010). A direct covalent interaction of a Michael acceptor pharmacophore of parthenolide with cysteine residues in some proteins important for melanoma development might therefore be responsible for effects triggered by parthenolide. One of the proteins, the activity of which could be decreased by Michael type covalent modification is IKKβ, a kinase activating transcription factor NF-κB (Wang et al., 2006).
The major aim of the current study was to investigate the effects of parthenolide on NF-κB activity in melanoma cells either untreated or treated with cisplatin. The cutaneous melanoma cell lines from the vertical growth phase (VGP) with competence for metastasis (WM793), the lung variant of WM793 established in nude mice (1205Lu) and highly aggressive melanoma cells (A375), all with constitutive NF-κB activity, were used in this investigation. Our results showing the inhibitory effect of parthenolide on NF-κB activity prompted us to study its effects on NF-κB downstream pathways leading to changes in cell cycle progression, migration, invasiveness and apoptotic response. Although the work presented here strongly indicates that parthenolide could act as an inhibitor of NF-κB pathway, the diversity of response to this drug observed in three melanoma cell lines suggests that NF-κB pathway could be considered as only one of the potential targets of parthenolide in melanoma cells.
Methods
Cell lines and drugs
WM793B cells, obtained from ATCC (American Type Culture Collection; Manassas, VA, USA), were maintained in 2% medium containing a (4:1) mixture of MCDB153 medium with 1.5 g·L−1 bicarbonate and Leibovitz L-15 medium with 2 mM l-glutamine, 0.005 mg·mL−1 bovine insulin, 1.68 mM CaCl2, 2% FBS (fetal bovine serum) and antibiotics. A375 and 1205Lu cell lines (a gift from Prof Piotr Laidler, Jagiellonian University, Poland) were maintained in RPMI1640 medium supplemented with 10% FBS and antibiotics. Neonatal human epidermal melanocytes (NHEM; Clonetics Melanocyte Cell Systems from Cambrex Bio Science, Walkersville, MD, USA) were maintained in medium used for culturing of WM793 cells with supplements for melanocytes (Provitro, Berlin, Germany). For experiments, medium was substituted with fresh medium containing 0.5% FBS. Parthenolide (BIOMOL International, Exeter, UK) was dissolved in dimethyl sulphoxide (DMSO). An equivalent concentration of DMSO was used in the control cultures. Cisplatin (EBEWE, Unterach, Austria) was dissolved in culture medium immediately before use. Treatment with tumour necrosis factor (TNFα; 20 ng·mL−1; Sigma-Aldrich, St. Louis, MO, USA) was carried out for 1 h.
Nuclear extract preparation and electrophoretic mobility shift assay (EMSA)
Melanoma cells were grown for 3 days. After preliminary time-response experiments, cell cultures were treated with parthenolide or vehicle (DMSO) for 3 h at indicated concentrations or pretreated with parthenolide for 1 h, and then treated with cisplatin for 2 h. In some experiments, cells were stimulated with TNFα (20 ng·mL−1) for 1 h. Nuclear extracts were prepared from adherent cells as described previously (Moll et al., 1995). Double-stranded DNA oligonucleotide containing NF-κB-binding sequence (5′-AAT TAG TTG AGG GGA CTT TCC CAG GC-3′) was [α-32P]dATP end-labelled using Sequenase 2.0 (USB Corporation, Cleveland, OH, USA), and purified on 7% PAGE. Labelled oligonucleotide (100 000 cpm), 15 µg of nuclear extract proteins, 2 µg of poly[dIdC]·[poly[dIdC] were incubated in binding buffer (Szulawska et al., 2005) for 20 min. After electrophoresis on native 5% polyacrylamide gels in 0.5 × TBE buffer, vacuum-dried gels were visualized by Molecular Imager® FX (Bio-Rad, Hercules, CA, USA). The specificity of the protein-DNA complexes was confirmed by competition experiments with a non-radioactive oligonucleotide with NF-κB-binding site (specific competitor), or an oligonucleotide with unrelated sequence (Sp1-binding site: 5′-ATT CCC CGC CCC CGC CCC C-3′). For the supershift experiments, nuclear extracts were pre-incubated with 4 µg of appropriate antibodies purchased from Santa Cruz Biotechnology (CA, USA): anti-p50 (sc-7178 X) and anti-p65 (sc-372 X), and IgG (sc-2027). The densitometric analysis of EMSA results was performed using Quantity One version 4.4.1. Software (Bio-Rad, Hercules, CA, USA).
RNA, primers and quantitative real-time PCR
Total RNA and cDNA were prepared as described before (Szulawska et al., 2007). Amplification reactions were performed using Kapa SYBR Fast qPCR kit (Kapa Biosystems, Cape Town, South Africa), 250 nM primers and 25 ng DNA template per reaction. The primers used for real-time were following: cyclin D1: 5′-CCC GCA CGA TTT CAT TGA AC-3′ and 5′-AGG GCG GAT TGG AAA TGA AC-3′; interleukin-8 (IL-8): 5′-AGG TGC AGT TTT GCC AAG GA-3′ and 5′-TTT CTG TGT TGG CGC AGT GT-3′; matrix metalloproteinase 9 (MMP-9): 5′-GAC CAG GAC AAG CTC TAC GG-3′ and 5′-CAG AAG CCC CAC TTC TTG TC-3′; Bcl-XL: 5′-GGC GGA TTT GAA TCT CTT TCT C-3′ and 5′-TTA TAA TAG GGA TGG GCT CAA CC-3′; survivin: 5′-GCT TTC AGG TGC TGG TAG-3′ and 5′-GAT GTG GAT CTC GGC TTC-3′. Gene expression levels were tested using the Rotor-Gene 3000 Real-Time DNA analysis system (Corbett Research, Morklake, Australia). The relative expression was based on the expression ratio of the target genes versus a reference gene RPS17 (QuantiTect Primer Assay, Qiagene, Valencia, CA, USA). To calculate the relative gene expression ratios, a mathematical model was used which included an efficiency correction for real-time PCR.
Cell cycle analysis
Melanoma cells were treated with vehicle or drugs at the indicated concentrations for 24 h, harvested by trypsinization and stained with propidium iodide (PI). The cell cycle profiles were obtained by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA, USA). ModFit LT 3.0 software (Verify Software, Topsham, MN, USA) was used to calculate the percentages of cells in each cycle phase.
Flow cytometric analysis of cell death and dissipation of mitochondrial membrane potential
In each of the following assays, cells were treated with drugs at the indicated concentrations for 22 h, except for viability assay where 4 h treatment was used in addition. Adherent and floating cells were combined. Flow cytometry was used to assess the percentages of PI-negative cells, which were then shown as viability (%). The detection of apoptotic and necrotic cells was carried out by dual staining with FITC-conjugated Annexin V and PI (Roche Diagnostics, Mannheim, Germany) as described before (Sztiller-Sikorska et al., 2009). To monitor changes in mitochondrial membrane potential (ΔΨm), cells were loaded with tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes, Invitrogenen, Eugene, OR, USA). All flow cytometry analyses were performed using FACSCalibur. Results were processed by using CellQuest software (Becton Dickinson).
Acridine orange/ethidium bromide staining
Cells treated with drugs for 22 h were stained with acridine orange (AO) and ethidium bromide (EB) (Sigma-Aldrich), and examined by fluorescence microscopy (Olympus BX41, Olympus Optical Co, London, UK) as described before (Czyz et al., 2008). In each experiment, more than 200 cells were analysed and then percentages of apoptotic or necrotic cells were calculated.
Migration and invasion assays
Mitomycin (5 µg·mL−1; Sigma-Aldrich) was used in confluent cultures for 2 h to inhibit proliferation. Multiple uniform streaks were made on the monolayer cultures, and the pictures were taken at 0 h and after 10 h of incubation in medium containing 0.5% FBS and drugs at the indicated concentrations. A digital camera (Olympus C-5050) attached to an inverted phase contrast microscope (Olympus CKX41) was used. Five to eight fields were analysed (about 300 cells in control culture) in each experiment. Cells in the middle of the scratched area were not counted.
The cell invasion assay was performed using BD BioCoat™ Matrigel Invasion Chambers (BD Biosciences, Bedford, MA, USA) with an 8 µm pore size PET membrane. Nearly confluent cultures of melanoma cells were either treated with vehicle (DMSO) or drugs for 3 h. After trypsinization, 50 000 cells were seeded in Matrigel invasion chambers. After 17 h of additional incubation with drugs, non-invading cells were removed from the upper surface of the membrane, and the cells on the lower surface were fixed with ethanol and stained with haematoxylin and eosin, photographed under the microscope (Olympus BX41), and counted in at least 10 fields.
Statistical analysis
Data represent means ± SD from at least three separate experiments. The significance of a difference in mean values for any tested parameter was validated by a Student's paired t-test. The difference was considered significant if P < 0.05.
Results
Parthenolide inhibits constitutive and induced NF-κB activity in melanoma cells
NF-κB DNA-binding activity was observed in all tested melanoma cell lines as shown by EMSA (Figure 1A). In untreated melanoma cells, the lowest level of NF-κB activity was observed in WM793 cells. Parthenolide inhibited the activity of NF-κB in a dose-dependent manner, most efficiently at a high concentration of 24 µM given for 3 h, although a loss of binding activity was also observed at lower concentrations. Parthenolide did not decrease cell viability at this time point (Figure 1D, open symbols). Shorter (2 h) or longer (20 h) incubation time did not intensify the effect of parthenolide on NF-κB activity (not shown). The A375 cells were the least sensitive, as 12 µM parthenolide reduced intensity of the NF-κB band only to about 75% of that in the control cells, whereas for WM793 and 1205Lu cells the reduction was to approximately 41% and 37% respectively (Figure 1B). IC50 values for the inhibition of NF-κB activity were calculated (Table 1). A significant stimulation of NF-κB activity was observed in TNFα-treated A375 and WM793 cells but not in 1205Lu cells (Figure 1C). Cisplatin slightly increased the level of NF-κB activity but only in A375 cells (Figure 1C). To determine the combined effect of parthenolide and cisplatin on NF-κB activity, cells were pretreated with 24 µM parthenolide for 1 h and then treated with 24 µM parthenolide and 5 µM cisplatin for additional 2 h. NF-κB activity was diminished by this combined treatment to a similar level as in cells treated with parthenolide alone. These results demonstrate that parthenolide is an inhibitor of constitutive, as shown for all three cell lines, as well as cisplatin-induced NF-κB activity in A375 cells.
Figure 1.
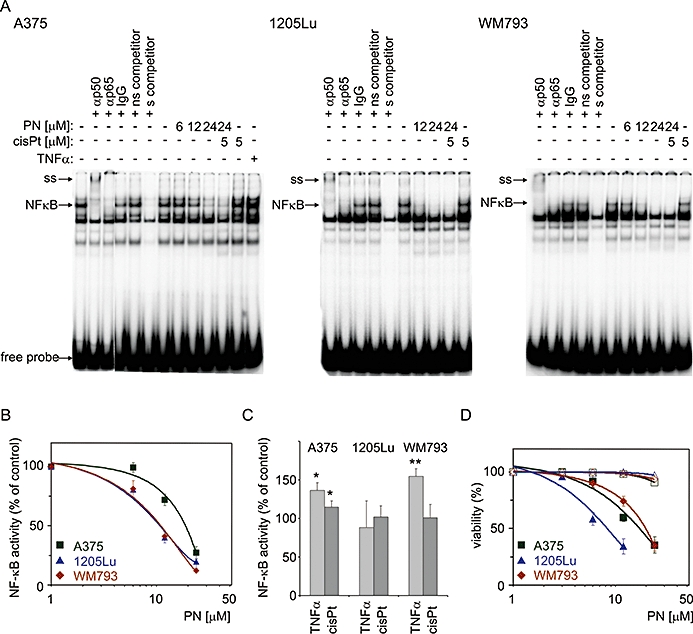
Parthenolide attenuated NF-κB activity in untreated and cisplatin-treated melanoma cells. Confluent cultures of A375, 1205Lu and WM793 cells were incubated with parthenolide (PN) or cisplatin (cisPt). In combined treatment, cells were pretreated with 24 µM parthenolide for 1 h, and then 5 µM cisplatin was added and incubation was continued for 2 h. In some experiments, cells were stimulated with TNFα (20 ng·mL−1) for 1 h as shown for A375 cells. To confirm NF-κB-binding specificity, competition assay was conducted with 100-fold excess of non-labelled NF-κB oligonucleotide (s), and non-specific (ns) oligonucleotide (Sp1-binding site). (A) supershift experiment with anti-p50 or anti-p65 antibodies was included. IgG antibody was used as a negative control. The arrows indicate the NF-κB-DNA-specific complex (NF-κB) and supershift (ss). (B) The NF-κB activity was quantified by densitometric scanning and expressed as percentages of the activity obtained in untreated cells. Half maximal inhibitory concentration (IC50) values were calculated and are shown in Table 1. (C) Effects of cisplatin (5 µM) and TNFα (20 ng·mL−1) on NF-κB DNA-binding activity in tested melanoma cell lines. (D) Viability of melanoma cells, assessed by flow cytometry after 4 h and after 22 h treatment, with the indicated concentrations of parthenolide, is shown as percentage of PI-negative cells. The data are means ± SD of three independent experiments. NF-κB, nuclear factor kappa B; TNFα, tumour necrosis factor alpha.
Table 1.
Effects of parthenolide on melanoma cells in vitro
| Cell line | IC50 (µM) | Range of IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| Inhibition of NF-κB activity (% of control; 3 h) | Viability (% PI-negative cells; 22 h) | Apoptosis (% Annexin V-positive cells; 22 h) | ΔΨm dissipation (% low TMRE fluorescence; 22 h) | Cell migration (% of control; 10 h) | Invasiveness (% of control; 20 h) | |
| A375 | 17.5 | 15.9 | 11.6 | 6–12 | 6–12 | 3–6 |
| 1205 Lu | 12.0 | 7.9 | 5.6 | 3–6 | ND | ND |
| WM793 | 11.0 | 18.3 | 15.5 | 6–12 | 6.0 | <3a |
When invasiveness was increased by TNFα treatment, about 50% inhibition of invasiveness was obtained with 3 µM parthenolide.
ΔΨm, mitochondrial transmembrane potential; ND, not determined; NF-κB, nuclear factor kappa B; PI, propidium iodide; TMRE, tetramethylrhodamine ethyl ester; TNFα, tumour necrosis factor alpha.
Parthenolide represses NF-κB-dependent gene expression
The expression of some proliferative, anti-apoptotic and metastatic genes, which have NF-κB-binding sites in their promoters, was assessed by real-time PCR (Figure 2). As in EMSA experiments, parthenolide at 12 µM and 24 µM was used either alone or in combination with 5 µM cisplatin. For 1205Lu cells which appeared to be more sensitive to parthenolide treatment (Figure 1D), lower concentrations of the compound were included.
Figure 2.
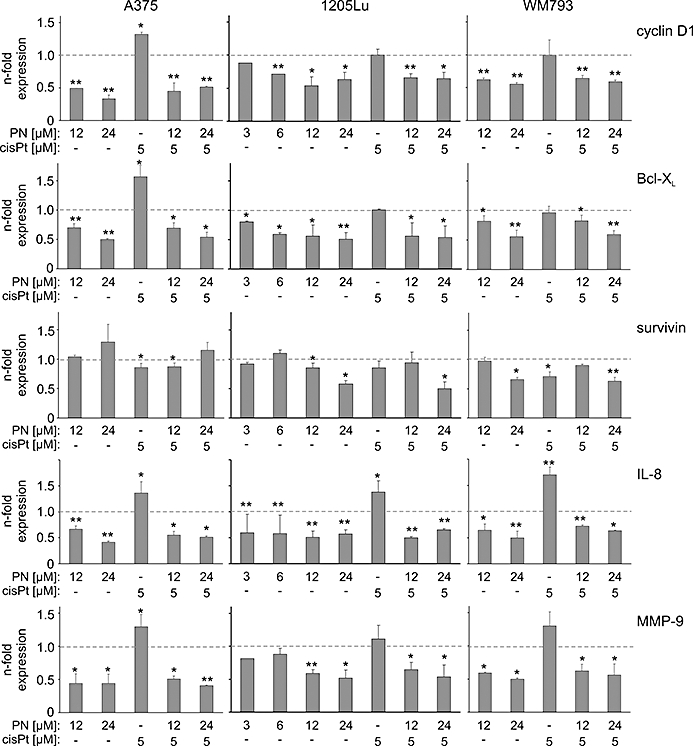
Expression levels of NF-κB-dependent genes, also those enhanced by cisplatin (cisPt) treatment, were reduced in melanoma cells treated with parthenolide (PN). Decreased mRNA levels in A375, 1205Lu and WM793 cells upon 4 h (Bcl-XL, cyclin D1) or 16 h (survivin, IL-8, MMP-9) drug treatment were analysed with real-time PCR as described in Materials and Methods. Data represent means ± SD of at least three independent experiments (*P < 0.05, **P < 0.01). Bcl-XL, Bcl-2-related gene, long isoform; IL-8, interleukin 8; MMP-9, matrix metalloproteinase 9.
Cyclin D1, a key cell cycle regulator, is over-expressed in melanoma cells. NF-κB controls cyclin D1 expression through direct transcriptional regulation mediated by several NF-κB DNA-binding sites in the promoter region. Parthenolide at 12 µM significantly decreased cyclin D1 expression in all tested cell lines, whereas 5 µM cisplatin induced an opposite effect but only in A375 cells. When parthenolide was used together with cisplatin, the mRNA level of cyclin D1 was decreased to a similar extent as in parthenolide-treated cells. Exposure of melanoma cells to parthenolide affected the expression of the anti-apoptotic genes, Bcl-XL and survivin. Parthenolide decreased the mRNA level of Bcl-XL and, in combined treatment with cisplatin, it counteracted a significant increase of Bcl-XL expression caused by cisplatin in A375 cells. Survivin, an inhibitor of apoptosis and mitotic regulator, is over-expressed in melanoma cells. In our study, the expression of survivin was markedly affected by parthenolide in 1205Lu and W793 cells, but not in A375 cells. NF-κB is also necessary for synthesis of the angiogenic factor interleukin(IL)-8. Parthenolide at 6 µM markedly reduced expression of IL-8, and this effect was enhanced at higher concentrations (Figure 2). Expression of IL-8 was strongly up-regulated in response to 5 µM cisplatin, and this effect was also significantly reduced by parthenolide. The mRNA level of MMP-9, which is linked to invasion, was affected by parthenolide and cisplatin. Parthenolide significantly reduced, whereas cisplatin increased, expression of MMP-9 in all cell lines. When used in combination with cisplatin, parthenolide diminished MMP-9 gene expression to a similar level as when used alone.
In contrast to WM793 cells, NF-κB activity was not significantly changed in 1205Lu cells upon TNFα stimulation (Figure 1C). Therefore, we evaluated changes in mRNA level of Bcl-XL and MMP-9 after 1 h TNFα treatment. In 1205Lu cells, TNFα slightly but significantly decreased their expression levels to 0.79 ± 0.05 and 0.71-fold control respectively. As expected, WM793 cells responded differently to TNFα treatment with increased expression of Bcl-XL (1.12 ± 0.07-fold control) and MMP-9 (1.14 ± 0.04-fold control).
In summary, 5 µM cisplatin markedly increased the expression of IL-8 and MMP-9 in all tested cell lines, whereas Bcl-XL and cyclin D1 expression was only increased in A375 cells. Parthenolide at 12 µM significantly reduced expression of all tested genes except for survivin in A375 and WM793 cells.
Diverse effects of parthenolide and cisplatin on cell cycle progression in tested melanoma cells and melanocytes
Expression of cyclin D1, which is important in the G1/S transition, was decreased by parthenolide in all tested cell lines (Figure 2). Therefore, cell cycle analysis was performed in adherent cells exposed to parthenolide for 24 h (Figure 3A). Parthenolide at 6 µM induced growth arrest in Go/G1 phase in WM793 cells, whereas in 1205Lu cells, at this concentration, it generated mainly hypodiploid cells. To show cell cycle arrest in Go/G1 phase in 1205Lu cells, a lower concentration of parthenolide was used (Figure 3B). Although cyclin D1 expression in A375 cells was also reduced by parthenolide (Figure 2), these cells did not accumulate in G0/G1 after this treatment (Figure 3A), which suggested that the G1/S transition in this case was cyclin D1-independent.
Figure 3.
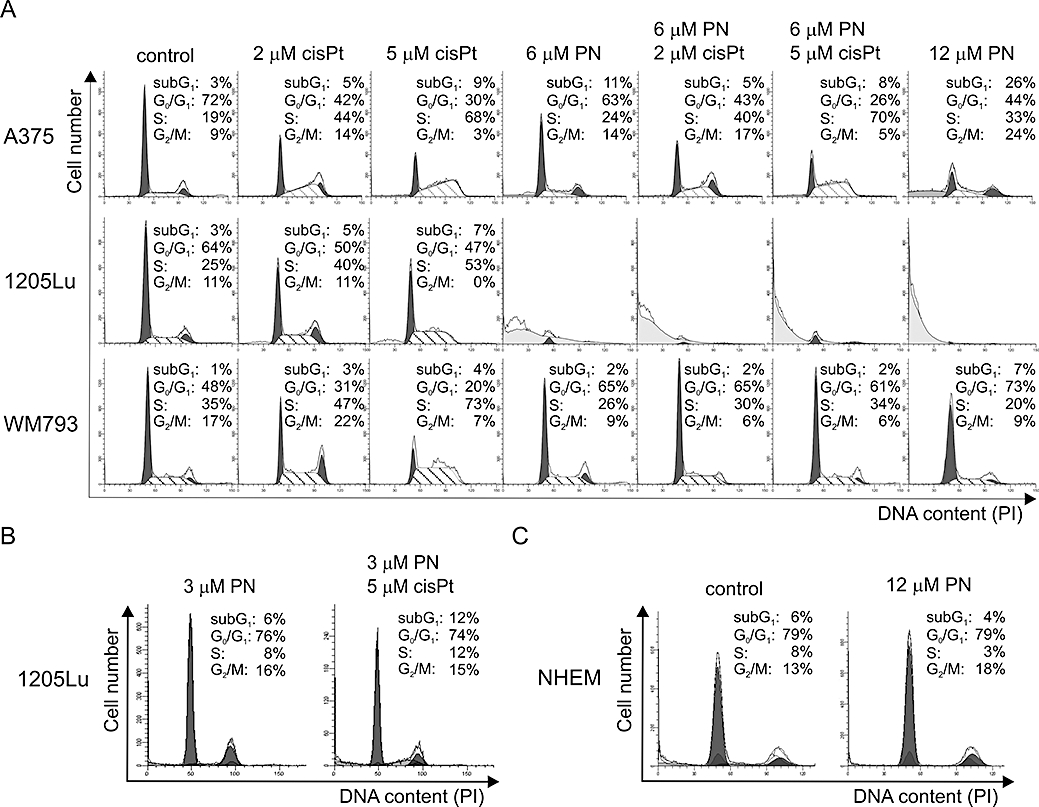
Diverse effects of parthenolide (PN) and/or cisplatin (cisPt) on cell cycle progression in A375, 1205Lu, WM793 cells and normal melanocyte (NHEM) cultures. (A) Distribution of the adherent cells through the cell-cycle phases was analysed by flow cytometry in adherent cells treated with drugs or vehicle for 24 h. Representative histograms and data from three independent experiments are shown. To calculate percentages of the cells in each fraction ModFit LT 3.0 software was used. For the parthenolide-treated 1205Lu cells, it was not possible to obtain valid data because of the high percentage of cells in the hypodiploid fraction. Therefore, parthenolide at lower concentration (3 µM) was used either alone or in combination with 5 µM cisplatin (B). (C) Parthenolide at 12 µM did not affect distribution of normal melanocytes (NHEM) in cell cycle and did not increase their accumulation in subG1 phase. NHEM, neonatal human epidermal melanocytes.
Cisplatin at 5 µM accumulated melanoma cells in S phase. This effect was clearly visible in all cell lines (Figure 3A). However, when 1205Lu and WM793 cells were exposed to combined treatment with parthenolide and cisplatin, they were arrested in G0/G1 phase. In turn, the accumulation of A375 cells in S phase after combined treatment was similar as that observed for cisplatin treatment, which confirmed above results showing no substantial influence of 6 µM parthenolide on A375 cell cycle profile.
When cell cycle profiles were generated for normal melanocytes (NHEM) treated with 12 µM parthenolide for 24 h, no signs of cell death and no changes in the distribution in cycle phases were observed (Figure 3C) indicating that parthenolide, even at that high concentration, was not lethal to normal melanocytes.
Parthenolide promotes apoptosis in melanoma cells via disruption of mitochondrial function
It has already been shown that constitutive activity of NF-κB in melanoma cells, which can be further enhanced by the treatment with anti-cancer drugs, leads to a strong anti-apoptotic signal. To test whether parthenolide would increase apoptosis in cisplatin-treated melanoma cells, we employed flow cytometric analysis of Annexin V/PI-stained cells (Figure 4A), and fluorescence microscopy for acridine orange/ethidium bromide (AO/EB)-stained cells (Figure 4B). Cisplatin at 2 or 5 µM did not induce apoptosis. About 80% of 1205Lu cells treated with 6 µM parthenolide were Annexin V-positive, and showed morphological features of apoptosis in AO/EB dual staining. A375 and WM793 cells were much less affected. To search for an explanation for the differences in apoptotic response between tested cell lines, we compared the relative expression of two anti-apoptotic genes Bcl-XL and survivin in untreated melanoma cells, taking the expression of those genes in A375 cells as a reference. In comparison with the mRNA level observed in untreated A375 cells, untreated 1205Lu cells had much lower level of Bcl-XL mRNA (0.57 ± 0.14-fold, P < 0.005), whereas the expression of survivin was the highest in untreated WM793 cells (2.69 ± 1.16-fold, P < 0.05). The differences in basal levels of Bcl-XL and survivin mRNAs observed in tested cell lines could partially explain why 1205Lu cells were the most, and WM793 cells were the least, sensitive to parthenolide.
Figure 4.
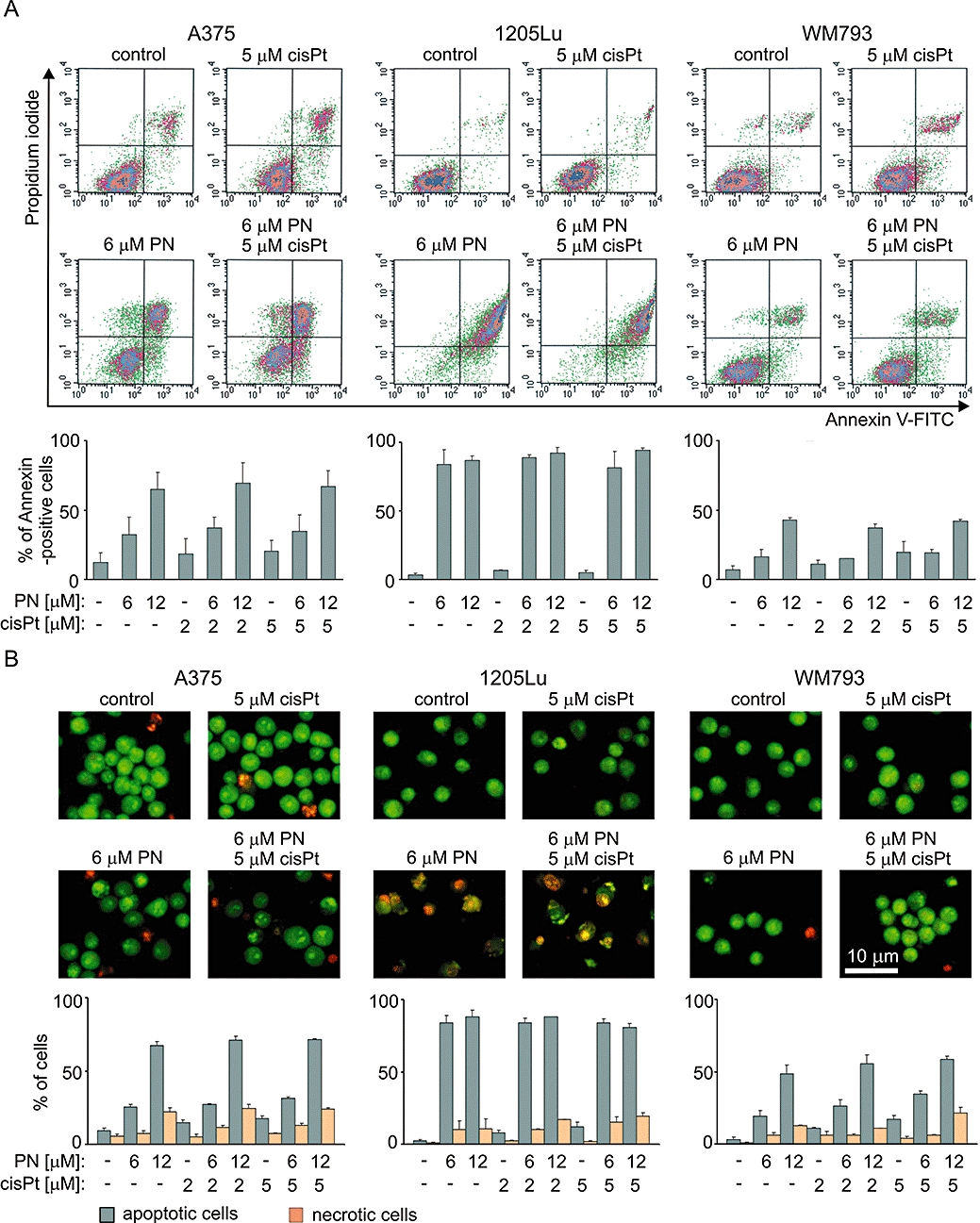
Parthenolide (PN) but not cisplatin (cisPt) at low concentrations induced apoptosis in melanoma cells. (A) Externalization of phosphatidylserine was determined by Annexin V/PI staining and flow cytometric analysis. One representative of three independent experiments and bars showing average percentages of Annexin V-positive cells. (B) Dual staining (AO/EB) of melanoma cells. Representative microscopic fields and quantitative data presenting percentages of apoptotic and necrotic cells are shown. The data are means ± SD of three independent experiments (P < 0.05, except for cisplatin treatment where no significant differences vs. control were obtained). AO, acridine orange; EB, ethidium bromide; PI, propidium iodide.
Anti-apoptotic members of the Bcl-2 family maintain the integrity of the mitochondrial membrane. The observed decrease in gene expression of Bcl-XL after parthenolide treatment (Figure 2) prompted us to investigate the effects of parthenolide on mitochondrial transmembrane potential (ΔΨm). In untreated and cisplatin-treated cultures, the majority of cells was within the population with high ΔΨm (Figure 5). Parthenolide caused a significant dissipation of ΔΨm. In 1205Lu cells, parthenolide at 6 µM was sufficient to decrease ΔΨm in about 87% of the cells, whereas in A375 and WM793 cells, a loss of ΔΨm was observed only in about 40% of the cells (Figure 5). Interestingly, at least additive effects on ΔΨm were observed when A375 and WM793 cells were treated simultaneously with 6 µM parthenolide and 5 µM cisplatin.
Figure 5.
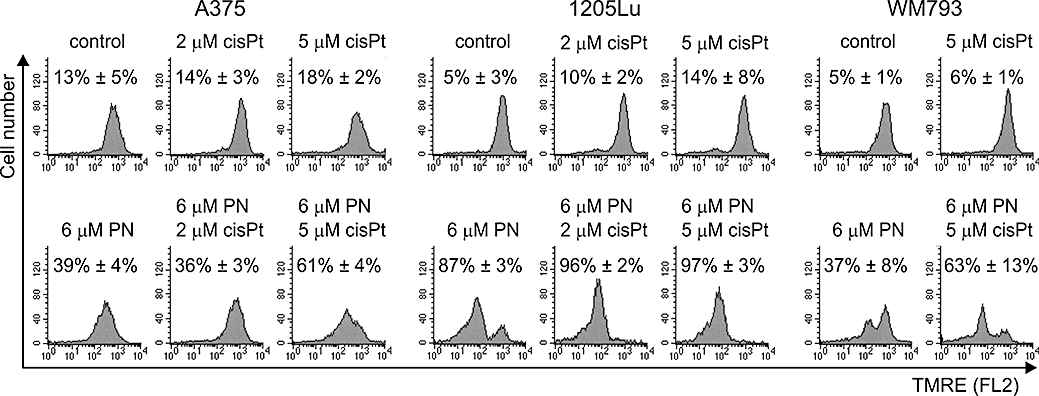
Loss of mitochondrial transmembrane potential (ΔΨm) was markedly increased in A375 and WM793 cells when 6 µM parthenolide (PN) was combined with cisplatin (cisPt). Cells were stained with TMRE and analysed by flow cytometry. Representative histograms from one typical experiment are shown. Data from three independent experiments are expressed as percentage of cells with low TMRE fluorescence. (P < 0.05, except for cisplatin treatment). TMRE, tetramethylrhodamine ethyl ester.
As 1205Lu cells were much more sensitive to parthenolide than two other melanoma cell lines (Figure 1D, Figure 3A, Figure 4A,B and Figure 5), we used a lower concentration of parthenolide to assess its effects on tested parameters in these cells. Parthenolide at 3 µM which arrested cells mainly in G0/G1 (Figure 3B), induced apoptosis in about 14% of cells (Figure 6A). The percentage of Annexin V-positive cells was increased when 3 µM parthenolide was combined with 5 µM cisplatin. Although the dissipation of ΔΨm was enhanced by the combined treatment, this effect was not statistically significant (Figure 6B).
Figure 6.
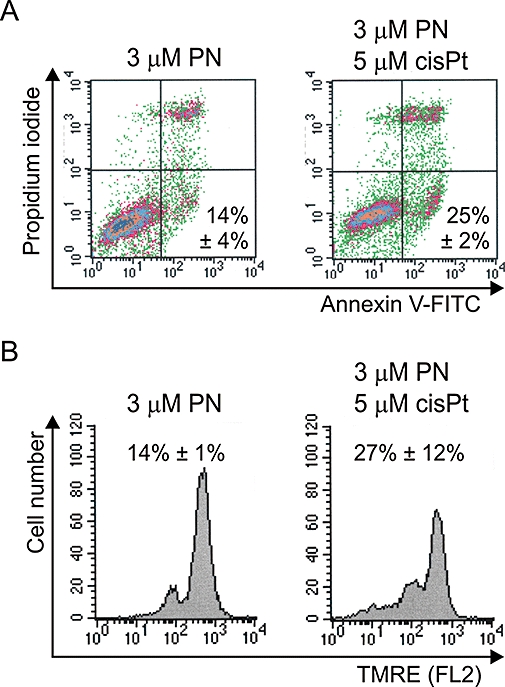
Low concentration effects of parthenolide (PN), alone or in combination with cisplatin (cisPt), in 1206Lu cells. (A) Apoptosis was assessed by flow cytometric analysis of Annexin V-FITC/PI-stained cells. Data from two independent experiments are expressed as average percentages of Annexin V-positive cells ± SD. (B) Changes in mitochondrial membrane potential were determined by flow cytometry after TMRE staining. Representative histograms from one typical experiment are shown. Data from two independent experiments are expressed as percentage of cells with low TMRE fluorescence ± SD. FITC, fluorescein isothiocyanate; PI, propidium iodide; TMRE, tetramethylrhodamine ethyl ester.
Parthenolide inhibits migration and invasion of melanoma cells
As MMP-9 expression in melanoma cells was significantly affected by parthenolide (Figure 2), migration and invasiveness were assessed. The migratory behaviour was determined by a scratch wound healing assay (Figure 7A). Migration was not affected by cisplatin. Parthenolide at 6 µM did not decrease migration of A375 cells, which had the highest migration capacity among untreated cultures, but the relative number of slowly migrating WM793 cells was reduced by 50%. 1205Lu cells became rounded and some of them detached from the plate surface during 10 h of incubation. Therefore, the results obtained for 1205Lu did not reflect parthenolide-reduced migration capacity but rather showed induction of apoptosis in those cells.
Figure 7.
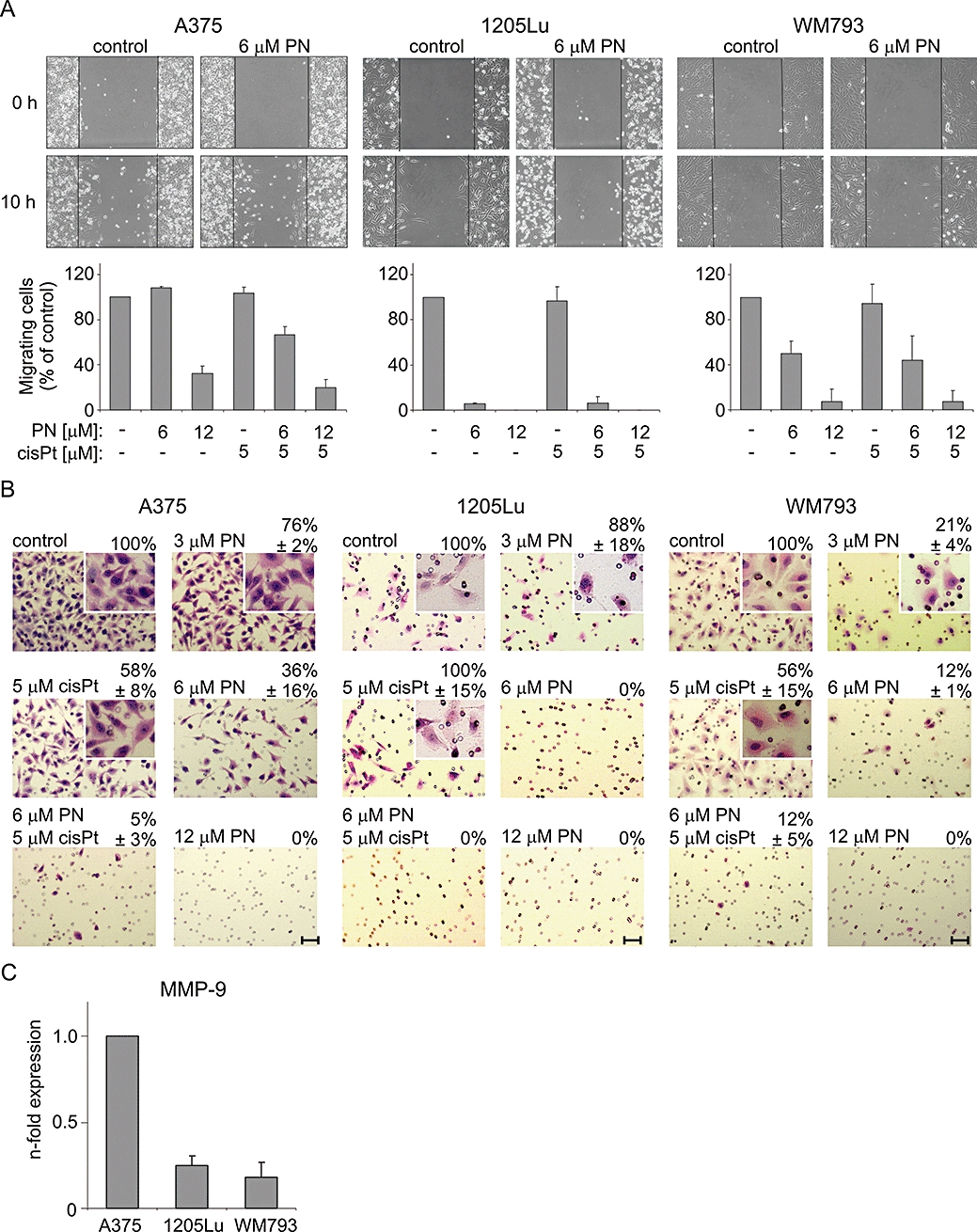
Effects of parthenolide (PN) and cisplatin (cisPt) on melanoma cell migration and invasiveness. (A) Parthenolide but not cisplatin reduced the migratory potential of WM793 and A375 cells, whereas results obtained in 1205Lu cells did not reflect the parthenolide-reduced migration capacity, due to increased cell death. Phase contrast microphotographs were taken after creation of wounds (0 h), and after 10 h of incubation of wounded cultures with parthenolide and/or cisplatin. About 300 migrating cells in untreated cultures were counted in 5–8 fields in each experiment. The same number of fields were used for counting cells in treated cultures, and their migratory potential was expressed as percentage of that obtained for control culture. Data are means ± SD of three independent experiments (P < 0.05, except for cisplatin treatment). (B) Parthenolide reduced the invasiveness of melanoma cells. Melanoma cell invasiveness was determined using a Matrigel-precoated cell culture inserts. Representative images from one typical experiment are shown. Scale bars, 10 µm. Magnification of insets was increased twice. (C) MMP-9 expression in untreated 1205Lu and WM793 cells in comparison with the expression in untreated A375 cells, measured by real-time PCR. Data represent means ± SD of three independent experiments (P < 0.01). MMP-9, matrix metalloproteinase 9.
The effects of parthenolide on the invasive potential of melanoma cells were determined using the Matrigel invasion assay. 1205Lu cells did not penetrate Matrigel to an extent sufficient for this analysis (Figure 7B), even when the incubation was prolonged to 28 h (not shown). Parthenolide decreased the invasiveness of A375 and WM793 cells in a concentration-dependent manner (Figure 7B). The number of invasive melanoma cells was significantly reduced after treatment with 3 µM parthenolide for 20 h. Parthenolide at 12 µM completely blocked invasion of A375 and WM793 cells through Matrigel. When the average numbers of untreated cells penetrating Matrigel were compared, it appeared that the invasiveness of untreated WM793 cells was 63% of that observed for untreated A375 cells. As differences in MMP-9 expression might contribute to the observed reduction of invasiveness, we assessed the relative expression levels of MMP-9 in all three cell lines. We found that the level of MMP-9 mRNA was about five times lower in untreated 1205Lu cells and about four times lower in untreated WM793 cells, than that in untreated A375 cells (Figure 7C). This could explain why the invasive potential of WM793 cells, which was already low in untreated cells, was strongly affected by parthenolide.
Parthenolide attenuates an increase in NF-κB activity and cell invasiveness triggered by TNFα in WM793 cells
We extended our study further and examined the suppression of TNFα-induced NF-κB activity by pretreatment with parthenolide. For this experiment, we chose WM793 cells with the lowest constitutive NF-κB activity. Indeed, TNFα, a well-known inducer of NF-κB transcription factor, significantly (by about 50%) increased NF-κB activity in WM793 cells (Figures 1C and 8A) without reducing cell viability (not shown). This was accompanied by increased invasiveness of WM793 cells (Figure 8B) and increased expression of MMP-9 (Figure 8C). Parthenolide prevented TNFα-induced NF-κB activation, invasiveness and MMP-9 expression in WM793 cells (Figure 8).
Figure 8.
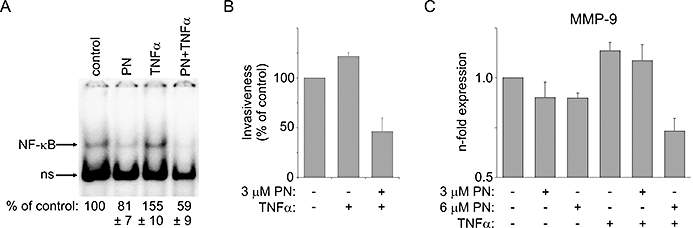
Parthenolide (PN) prevented TNFα-induced NF-κB activation, MMP-9 expression and invasiveness in WM793 cells. (A) TNFα (20 ng·mL−1)-induced NF-κB activity was reduced by 6 µM parthenolide. The NF-κB activity levels were estimated by densitometric scanning and shown as percentages of the activity level obtained in untreated cells ± SD (P < 0.05). Invasiveness (B) and MMP-9 expression (C) of WM793 cells increased by TNFα treatment were significantly reduced by parthenolide treatment (P < 0.01 from control, P < 0.05 from TNFα-stimulated). MMP-9, matrix metalloproteinase 9; NF-κB, nuclear factor kappa B; TNFα, tumour necrosis factor alpha.
Discussion and conclusions
Melanoma is not a uniform cancer. Diverse molecular changes underlying melanoma development need to be addressed for effective treatment (Chin et al., 2006; Yang et al., 2009; Smalley, 2010). An IKK/NF-κB module belongs to one of the important signalling pathways constitutively activated in melanoma cells. It provides protection from apoptosis and contributes to the chemoresistance of melanoma cells. Therefore, inhibitors of NF-κB pathway are considered as an adjuvant approach in melanoma treatment (Sweeney et al., 2005; Schön et al., 2008; Baud and Karin, 2009).
In the current study, we focused our attention on parthenolide, which is already recognized as an inhibitor of NF-κB (Bork et al., 1997). We investigated its effects on three well-defined melanoma cell lines, characterized by the BRAFV600E mutation, wild-type p53, and as shown by our study different basal constitutive NF-κB activity. Upon parthenolide treatment, the highly metastatic A375 cells were markedly affected in their ability to invade collagen matrix, whereas 1205Lu cells were efficiently stimulated to undergo apoptosis. VGP primary WM793 cells were the least sensitive, and mainly cell cycle arrest in G0/G1 phase was observed. These diverse outcomes suggest that parthenolide might be recognized as an example of a poly-pharmacological drug able to bind and modulate multiple targets in melanoma cells, and NF-κB pathway could be one of such targets.
Our experiments measuring the expression of NF-κB-dependent genes provided the mechanistic evidence for the diverse biological effects of parthenolide in melanoma cells. Constitutive NF-κB activation has been shown to promote oncogenic phenotype of melanoma cells partially via IL-8 expression (Peng et al., 2007), and increased serum concentration of IL-8 in melanoma patients correlates with poor prognosis (Ugurel et al., 2001). Expression of IL-8 in A375 cells was markedly reduced by another IKK inhibitor, KINK-1 (Schön et al., 2008). Our data provide evidence that parthenolide can reduce expression of IL-8 mRNA in melanoma cells, also in those treated with cisplatin. IL-8 downstream of the p38/NF-κB pathway is important for melanoma cell migration and proliferation, including A375 cells (Estrada et al., 2009). Therefore, the reduced IL-8 expression (Figure 2) could be partially responsible for decreased ability to migrate and penetrate collagen matrix observed in our study (Figure 7A and B). The extracellular matrix degradation involves a number of proteins including the MMPs. High and inducible expression of MMP-9 is observed in aggressive stages of melanoma, and its inhibition reduced the metastatic potential of melanoma cells (Huang et al., 2005). Inhibitory effects of parthenolide on tumour growth and MMP-9 protein level in a xenograft model of renal cell carcinoma were correlated with inhibition of NF-κB activation (Oka et al., 2007). In the current study, parthenolide suppressed mRNA expression of MMP-9 in metastatic A375 cells with high endogenous MMP-9 mRNA levels, and in primary WM793 cells with four times lower expression of that proteinase (Figure 7C). Parthenolide-induced inhibition of MMP-9 mRNA expression was shown in THP-1 macrophages (Dell’Agli et al., 2009) and W256 carcinosarcoma cells (Idris et al., 2009). Parthenolide reduced migration of mammary carcinosarcoma cells in vitro, and prevented osteolytic bone metastasis in vivo (Idris et al., 2009). The metastasis-preventing effects of parthenolide were observed in breast cancer cells (Sweeney et al., 2005) and osteosarcoma cells (Kishida et al., 2007). These findings together with our current results suggest that parthenolide is a promising candidate for an anti-metastatic drug.
Enhanced cyclin D1 expression is one of the regulatory mechanisms that melanoma cells use to overcome the G1 checkpoint. We found that parthenolide significantly decreased cyclin D1 expression in melanoma cells. This might contribute to accumulation of cells in G0/G1 phase in 1205Lu and WM793 cells (Figure 3). In addition, parthenolide might inhibit the transition from G1 to S phase via epigenetic regulation of p21 expression (Gopal et al., 2007). Parthenolide-induced G0/G1 phase cell cycle arrest was also observed in vascular smooth muscle cells (Weng et al., 2009) and human cholangiocarcinoma cells (Kim et al., 2005). In contrast, parthenolide induced G2/M arrest in hepatoma cells (Wen et al., 2002). This indicates that inhibition of the cell cycle phase might be cell-type-specific, and the cellular context-dependent influence of parthenolide on cyclin D1 expression might be one of the factors which can explain some of these differences.
Melanoma cells overexpress several anti-apoptotic proteins and the efficacy of anti-melanoma treatment depends on inhibition of expression of anti-apoptotic genes regulated by NF-κB (Yang et al., 2009). Our findings demonstrate that parthenolide could mediate its pro-apoptotic properties at two levels: upstream of mitochondria through down-regulation of Bcl-XL expression, and downstream through down-regulation of survivin expression. The differences between melanoma cell lines in the basal expression of Bcl-XL and survivin or other anti-apoptotic proteins, as well as their dependence on signalling pathways other than IKK/NF-κB, might contribute to the apoptotic response to parthenolide treatment observed in tested cell lines. The chemoresistant phenotype associated with melanomas could be the cooperative phenotype that results from activation of several pathways (Smalley, 2010). Their individual contributions and significance for overall drug resistance may differ markedly and the current results are compatible with this hypothesis. As summarized in Table 1, half maximal inhibitory concentration (IC50) values for NF-κB inhibition were similar in 1205Lu and WM793 cells, but parthenolide-induced changes in cell fate were different in these two cell lines. 1205Lu is the lung metastatic variant of WM793 established in nude mice. This could explain why having similar levels of NF-κB, these two cell lines responded almost identically to parthenolide in terms of inhibition of NF-κB activity. Different changes in viability of these cells suggest that NF-κB level was not the only factor determining cell death in response to parthenolide treatment. Some comparative genomic hybridization (CGH) and DNA microarray-based gene expression profiling analyses carried out in 1205Lu and WM793 cells (Gallagher et al., 2005), revealed that hypermethylation occurred with melanoma progression from the WM793 to the 1205Lu cells. As parthenolide was shown to be an inhibitor of DNA methyltransferase 1 (Liu et al., 2009), it is possible that parthenolide-induced hypomethylation in 1205Lu cells might contribute to effects specific for this cell line.
In addition to the direct effect of parthenolide on NF-κB activity, targeting this transcription factor could indirectly influence the pro-apoptotic activity of the tumour suppressor p53 (Kim et al., 2009). It is reasonable to assume that parthenolide, by inhibiting constitutive NF-κB activity, might simultaneously decrease repression of p53 pathway as inactive NF-κB is no longer able to up-regulate MDM2, or sequester p300 and CBP, or form a stable association of p65–p53 at the p53-responsive promoters to inhibit transcription. Moreover, it has recently been demonstrated (Gopal et al., 2009) that parthenolide could regulate p53 activity by promoting MDM2 ubiquitination. Parthenolide, by inhibiting NF-κB and activating p53 could therefore affect simultaneously two pathways crucial for cancer development. This is of importance as in most cases of melanoma, p53 is expressed in its wild-type configuration and NF-κB activity is constitutive.
In conclusion, we have found that parthenolide can induce anti-metastatic and pro-apoptotic effects in melanoma cells, that these effects, although not exclusively, are associated with the suppression of NF-κB activity and that parthenolide can counteract the cisplatin-induced or TNFα-triggered up-regulation of some NF-κB-dependent genes. The underlying mechanisms include down-regulation of genes expressing the anti-apoptotic proteins Bcl-XL and survivin, as well as cyclin D1, MMP-9 and IL-8. Finally, the diversity of outcomes in three melanoma cell lines suggests cell context-dependent, poly-pharmacological properties of parthenolide.
Our results clearly indicate that parthenolide could be therapeutically exploited in melanoma. In addition to the long history of medical application of Tanacetum parthenium, a plant where parthenolide is a major bioactive component, this compound has already been shown to be safe in Phase I/II clinical trials (Murphy et al., 1988; Curry et al., 2004). It is, however, important to note that the studies on the bioavailability of parthenolide are not conclusive yet (Sweeney et al., 2005; Dell’Agli et al., 2009). Parthenolide was shown to be toxic to hepatoma and leukaemia cells but not to normal liver and haematopoietic cells (Wen et al., 2002; Guzman et al., 2005), and as revealed by our study also non-toxic to normal melanocytes. Parthenolide could be considered for prophylactic treatment after surgery of primary melanoma lesions to prevent their metastasis. Used for centuries for migraine treatment, it is apparently able to cross the blood–brain barrier. As 30% of metastatic melanoma patients have metastases in the brain, where surgical interventions are of limited use, parthenolide might be of value in preventing or reducing such metastases. A protective function of parthenolide on renal tissue observed during cisplatin chemotherapy (Francescato et al., 2007) warrants its evaluation in combination with other anti-cancer agents. However, recently, parthenolide acted as an inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) and induced resistance to doxorubicin in human colon cancer cells (Riganti et al., 2009). Therefore, the possibility that parthenolide might reduce the efficacy of some anti-cancer drugs must be borne in mind. Future investigations to determine the combined effects of parthenolide and anti-cancer drugs in animal models and clinical trials are necessary to validate the usefulness of parthenolide as an adjuvant and also as part of a more personalized combination therapy for melanoma patients.
Acknowledgments
The authors wish to thank Dr Marta Stasiak for her help with flow cytometric analysis, Drs Malgorzata Sztiller-Sikorska, Justyna Jakubowska and Markus Düchler for stimulating discussions, Dr Dave Cavanagh for reviewing the manuscript, and Mrs Grazyna Kus for technical support. This research was supported by Grant N N401 020 235 from the Ministry of Scientific Research and Higher Education.
Glossary
Abbreviations:
- ΔΨm
mitochondrial transmembrane potential
- AO
acridine orange
- ATCC
American Type Culture Collection
- Bcl-XL
Bcl-2-related gene, long isoform
- DMSO
dimethyl sulphoxide
- DNMT1
DNA methyltransferase 1
- EB
ethidium bromide
- EMSA
electrophoretic mobility shift assay
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HDAC1
histone deacetylase 1
- IgG
immunoglobulin G
- IKK
IkappaB kinase
- IL-8
interleukin 8
- KINK-1
inhibitor of IKKβ
- MDM2
murine double minute 2
- MMPs
matrix metalloproteinases
- MMP-9
matrix metalloproteinase 9
- MTT
thiazolyl blue tetrazolium bromide
- NF-κB
nuclear factor kappa B
- NHEM
neonatal human epidermal melanocytes
- ns
non-specific
- PI
propidium iodide
- ROS
reactive oxygen species
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
- Sp1
specificity protein 1
- ss
supershift
- TMRE
tetramethylrhodamine ethyl ester
- TNFα
tumour necrosis factor alpha
- VGP
vertical growth phase
Conflict of interest
The authors state no conflict of interest.
References
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya LM, Abad MJ, Bermejo P. The role of parthenolide in intracellular signalling processes: review of current knowledge. Curr Sign Transd Ther. 2008;3:82–87. [Google Scholar]
- Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- Cory AH, Cory JG. Lactacystin, a proteasome inhibitor, potentiates the apoptotic effect of parthenolide, an inhibitor of NFkappaB activation, on drug-resistant mouse leukemia L1210 cells. Anticancer Res. 2002;22:3805–3809. [PubMed] [Google Scholar]
- Curry EA, 3rd, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004;22:299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- Czyz M, Jakubowska J, Sztiller-Sikorska M. STI571/doxorubicin concentration-dependent switch for diverse caspase actions in CML cell line K562. Biochem Pharmacol. 2008;75:1761–1773. doi: 10.1016/j.bcp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dell'Agli M, Galli GV, Bosisio E, D’Ambrosio M. Inhibition of NF-kB and metalloproteinase-9 expression and secretion by parthenolide derivatives. Bioorg Med Chem Lett. 2009;19:1858–1860. doi: 10.1016/j.bmcl.2009.02.080. [DOI] [PubMed] [Google Scholar]
- Duechler M, Stańczyk M, Czyz M, Stepnik M. Potentiation of arsenic trioxide cytotoxicity by Parthenolide and buthionine sulfoximine in murine and human leukemic cells. Cancer Chemother Pharmacol. 2008;61:727–737. doi: 10.1007/s00280-007-0527-3. [DOI] [PubMed] [Google Scholar]
- Estrada Y, Dong J, Ossowski L. Positive crosstalk between ERK and p38 in melanoma stimulates migration and in vivo proliferation. Pigment Cell Melanoma Res. 2009;22:66–76. doi: 10.1111/j.1755-148X.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- Fonrose X, Ausseil F, Soleilhac E, Masson V, David B, Pouny I, et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67:3371–3378. doi: 10.1158/0008-5472.CAN-06-3732. [DOI] [PubMed] [Google Scholar]
- Francescato HD, Costa RS, Scavone C, Coimbra TM. Parthenolide reduces cisplatin-induced renal damage. Toxicology. 2007;230:64–75. doi: 10.1016/j.tox.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Gallagher WM, Bergin OE, Rafferty M, Kelly ZD, Nolan IM, Fox EJ, et al. Multiple markers for melanoma progression regulated by DNA methylation: insights from transcriptomic studies. Carcinogenesis. 2005;26:1856–1867. doi: 10.1093/carcin/bgi152. [DOI] [PubMed] [Google Scholar]
- García-Piñeres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA-binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem Biol. 2007;14:813–823. doi: 10.1016/j.chembiol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gopal YN, Chanchorn E, Van Dyke MW. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther. 2009;8:552–562. doi: 10.1158/1535-7163.MCT-08-0661. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, et al. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol Cancer Ther. 2009;8:2339–2347. doi: 10.1158/1535-7163.MCT-09-0133. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, et al. Effects of the sesquiterpene lactone parthenolide on prostate tumour-initiating cells: an integrated molecular profiling approach. Prostate. 2009;69:827–837. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Giese A, Deppert W. Wild-type p53 in cancer cells : when a guardian turns into a blackguard. Biochem Pharmacol. 2009;77:11–20. doi: 10.1016/j.bcp.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Kim JH, Liu L, Lee SO, Kim YT, You KR, Kim DG. Susceptibility of cholangiocarcinoma cells to parthenolide-induced apoptosis. Cancer Res. 2005;65:6312–6320. doi: 10.1158/0008-5472.CAN-04-4193. [DOI] [PubMed] [Google Scholar]
- Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of Nuclear Factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- Lesiak K, Koprowska K, Zalesna I, Nejc D, Duchler M, Czyz M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anticancer activity against human melanoma cells in vitro. Melanoma Res. 2010;20:21–34. doi: 10.1097/CMR.0b013e328333bbe4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu WL, Guo J, Du J, Li T, Wu JW, et al. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Release. 2008;129:18–25. doi: 10.1016/j.jconrel.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, et al. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J Pharmacol Exp Ther. 2009;329:505–514. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–23485. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Bar-Eli M. Transcriptional control of the melanoma malignant phenotype. Cancer Biol Ther. 2008;7:997–1003. doi: 10.4161/cbt.7.7.6535. [DOI] [PubMed] [Google Scholar]
- Moll T, Czyz M, Holzmüller H, Hofer-Warbinek R, Wagner E, Winkler H, et al. Regulation of the tissue factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Sp1-like transcription factors. J Biol Chem. 1995;270:3849–3857. doi: 10.1074/jbc.270.8.3849. [DOI] [PubMed] [Google Scholar]
- Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet. 1988;2:189–192. doi: 10.1016/s0140-6736(88)92289-1. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumour agent parthenolide reverses resistance of breast cancer cells to tumour necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–7344. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, Nakayama M, et al. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res. 2007;313:551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D, et al. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1alpha and P-glycoprotein overexpression. Br J Pharmacol. 2009;156:1054–1066. doi: 10.1111/j.1476-5381.2009.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön M, Wienrich BG, Kneitz S, Sennefelder H, Amschler K, Vöhringer V, et al. KINK-1, a novel small-molecule inhibitor of IKKbeta, and the susceptibility of melanoma cells to antitumoural treatment. J Natl Cancer Inst. 2008;100:862–875. doi: 10.1093/jnci/djn174. [DOI] [PubMed] [Google Scholar]
- Smalley KSM. Understanding Melanoma signalling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37. doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- Sztiller-Sikorska M, Jakubowska J, Wozniak M, Stasiak M, Czyz M. A non-apoptotic function of caspase-3 in pharmacologically-induced differentiation of K562 cells. Br J Pharmacol. 2009;157:1451–1462. doi: 10.1111/j.1476-5381.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulawska A, Gniazdowski M, Czyz M. Sequence specificity of formaldehyde-mediated covalent binding of anthracycline derivatives to DNA. Biochem Pharmacol. 2005;69:7–18. doi: 10.1016/j.bcp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Szulawska A, Arkusinska J, Czyz M. Accumulation of gamma-globin mRNA and induction of irreversible erythroid differentiation after treatment of CML cell line K562 with new doxorubicin derivatives. Biochem Pharmacol. 2007;73:175–184. doi: 10.1016/j.bcp.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumour progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- Wang W, Adachi M, Kawamura R, Sakamoto H, Hayashi T, Ishida T, et al. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis. 2006;11:2225–2235. doi: 10.1007/s10495-006-0287-2. [DOI] [PubMed] [Google Scholar]
- Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- Weng SX, Sui MH, Chen S, Wang JA, Xu G, Ma J, et al. Parthenolide inhibits proliferation of vascular smooth muscle cells through induction of G0/G1 phase cell cycle arrest. J Zhejiang Univ Sci B. 2009;10:528–535. doi: 10.1631/jzus.B0820351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zaja-Milatovic S, Thu YM, Lee F, Smykla R, Richmond A. Molecular determinants of melanoma malignancy: selecting targets for improved efficacy of chemotherapy. Mol Cancer Ther. 2009;8:636–647. doi: 10.1158/1535-7163.MCT-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther. 2005;4:587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


