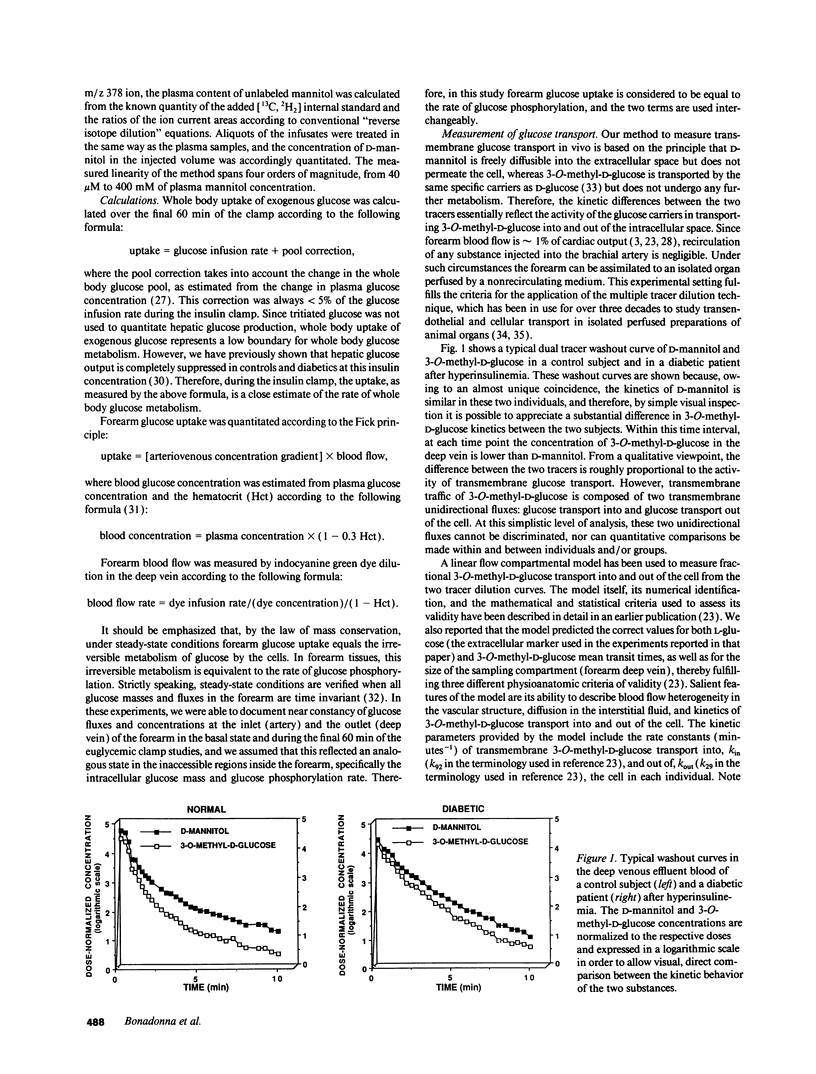
Abstract
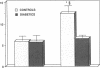
Insulin resistance for glucose metabolism in skeletal muscle is a key feature in non-insulin-dependent diabetes mellitus (NIDDM). Which cellular effectors of glucose metabolism are involved is still unknown. We investigated whether transmembrane glucose transport in vivo is impaired in skeletal muscle in nonobese NIDDM patients. We performed euglycemic insulin clamp studies in combination with the forearm balance technique (brachial artery and deep forearm vein catheterization) in six nonobese NIDDM patients and five age- and weight-matched controls. Unlabeled D-mannitol (a nontransportable molecule) and radioactive 3-O-methyl-D-glucose (the reference molecular probe to assess glucose transport activity) were simultaneously injected into the brachial artery, and the washout curves were measured in the deep venous effluent blood. In vivo transmembrane transport of 3-O-methyl-D-glucose in forearm muscle was determined by computerized analysis of the washout curves. At similar steady-state plasma concentrations of insulin (approximately 500 pmol/liter) and glucose (approximately 5.15 mmol/liter), transmembrane inward transport of 3-O-methyl-D-glucose in skeletal muscle was markedly reduced in the NIDDM patients (6.5 x 10(-2) +/- 0.56 x 10(-2).min-1) compared with controls (12.5 x 10(-2) +/- 1.5 x 10(-2).min-1, P < 0.005). Mean glucose uptake was also reduced in the diabetics both at the whole body level (9.25 +/- 1.84 vs. 28.3 +/- 2.44 mumol/min per kg, P < 0.02) and in the forearm tissues (5.84 +/- 1.51 vs. 37.5 +/- 7.95 mumol/min per kg, P < 0.02). When the latter rates were extrapolated to the whole body level, skeletal muscle accounted for approximately 80% of the defect in insulin action seen in NIDDM patients. We conclude that transmembrane glucose transport, when assessed in vivo in skeletal muscle, is insensitive to insulin in nonobese NIDDM patients, and plays a major role in determining whole body insulin resistance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson K., Galuska D., Thörne A., Sonnenfeld T., Wallberg-Henriksson H. Decreased insulin-stimulated 3-0-methylglucose transport in in vitro incubated muscle strips from type II diabetic subjects. Acta Physiol Scand. 1991 Jun;142(2):255–260. doi: 10.1111/j.1748-1716.1991.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Baly D. L., Horuk R. Dissociation of insulin-stimulated glucose transport from the translocation of glucose carriers in rat adipose cells. J Biol Chem. 1987 Jan 5;262(1):21–24. [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Saccomani M. P., Cobelli C., DeFronzo R. A. Effect of insulin on system A amino acid transport in human skeletal muscle. J Clin Invest. 1993 Feb;91(2):514–521. doi: 10.1172/JCI116230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Saccomani M. P., Seely L., Zych K. S., Ferrannini E., Cobelli C., DeFronzo R. A. Glucose transport in human skeletal muscle. The in vivo response to insulin. Diabetes. 1993 Jan;42(1):191–198. doi: 10.2337/diab.42.1.191. [DOI] [PubMed] [Google Scholar]
- COOPER K. E., EDHOLM O. G., MOTTRAM R. F. The blood flow in skin and muscle of the human forearm. J Physiol. 1955 May 27;128(2):258–267. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo B., Napoli R., Di Marino L., Picardi A., Riccardi G., Sacca L. Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab. 1988 Nov;67(5):893–898. doi: 10.1210/jcem-67-5-893. [DOI] [PubMed] [Google Scholar]
- Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990 Oct;70(4):1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- Clarys J. P., Martin A. D., Drinkwater D. T. Gross tissue weights in the human body by cadaver dissection. Hum Biol. 1984 Sep;56(3):459–473. [PubMed] [Google Scholar]
- Cobelli C., Saccomani M. P., Ferrannini E., Defronzo R. A., Gelfand R., Bonadonna R. A compartmental model to quantitate in vivo glucose transport in the human forearm. Am J Physiol. 1989 Dec;257(6 Pt 1):E943–E958. doi: 10.1152/ajpendo.1989.257.6.E943. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Dillon R. S. Importance of the hematocrit in interpretation of blood sugar. Diabetes. 1965 Oct;14(10):672–674. doi: 10.2337/diab.14.10.672. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Elton C. W., Friedman J. E., Pilch P. F., Pories W. J., Atkinson S. M., Jr, Caro J. F. Decreased expression of glucose transporter in muscle from insulin-resistant patients. Am J Physiol. 1991 Mar;260(3 Pt 1):E459–E463. doi: 10.1152/ajpendo.1991.260.3.E459. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Koranyi L., Bourey R., Schalin-Jäntti C., Widén E., Mueckler M., Permutt A. M., Groop L. C. Insulin resistance in type 2 (non-insulin-dependent) diabetic patients and their relatives is not associated with a defect in the expression of the insulin-responsive glucose transporter (GLUT-4) gene in human skeletal muscle. Diabetologia. 1992 Feb;35(2):143–147. doi: 10.1007/BF00402546. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Thuillez P., Lillioja S., Zawadzki J., Bogardus C. Insulin sensitivity in adipocytes from subjects with varying degrees of glucose tolerance. Am J Physiol. 1986 Sep;251(3 Pt 1):E306–E310. doi: 10.1152/ajpendo.1986.251.3.E306. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Hancock J. A., Golichowski A. M., Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992 Apr;41(4):465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991 Mar;87(3):1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Matthaei S., Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987 Jan 5;262(1):189–197. [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli G., Ferrannini E., Stern M., Haffner S., DeFronzo R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes. 1992 Dec;41(12):1575–1586. doi: 10.2337/diab.41.12.1575. [DOI] [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W. Qualitative and quantitative comparison of glucose transport activity and glucose transporter concentration in plasma membranes from basal and insulin-stimulated rat adipose cells. Biochem J. 1988 Jan 1;249(1):155–161. doi: 10.1042/bj2490155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler W. C., Pettitt D. J., Saad M. F., Bennett P. H. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990 Feb;6(1):1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- Kuikka J., Levin M., Bassingthwaighte J. B. Multiple tracer dilution estimates of D- and 2-deoxy-D-glucose uptake by the heart. Am J Physiol. 1986 Jan;250(1 Pt 2):H29–H42. doi: 10.1152/ajpheart.1986.250.1.H29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan R. J., Watson J. S., Weir J. The relative proportions of fat, muscle and bone in the normal human forearm as determined by computed tomography. Clin Sci (Lond) 1984 Jun;66(6):683–689. doi: 10.1042/cs0660683. [DOI] [PubMed] [Google Scholar]
- Mitrakou A., Kelley D., Veneman T., Jenssen T., Pangburn T., Reilly J., Gerich J. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes. 1990 Nov;39(11):1381–1390. doi: 10.2337/diab.39.11.1381. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen B. F., Hansen S. A. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J. 1988 Jun 15;252(3):733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. F., Knowler W. C., Pettitt D. J., Nelson R. G., Mott D. M., Bennett P. H. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988 Dec 8;319(23):1500–1506. doi: 10.1056/NEJM198812083192302. [DOI] [PubMed] [Google Scholar]
- Sasson S., Cerasi E. Substrate regulation of the glucose transport system in rat skeletal muscle. Characterization and kinetic analysis in isolated soleus muscle and skeletal muscle cells in culture. J Biol Chem. 1986 Dec 25;261(36):16827–16833. [PubMed] [Google Scholar]
- Sasson S., Edelson D., Cerasi E. In vitro autoregulation of glucose utilization in rat soleus muscle. Diabetes. 1987 Sep;36(9):1041–1046. doi: 10.2337/diab.36.9.1041. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Härkonen M., Groop L. C. Impaired activation of glycogen synthase in people at increased risk for developing NIDDM. Diabetes. 1992 May;41(5):598–604. doi: 10.2337/diab.41.5.598. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Raineri-Maldonado C., Buchanan C., Pories W. J., Carter-Su C., Pilch P. F., Caro J. F. Adipose tissue glucose transporters in NIDDM. Decreased levels of muscle/fat isoform. Diabetes. 1991 Apr;40(4):472–477. doi: 10.2337/diab.40.4.472. [DOI] [PubMed] [Google Scholar]
- Sternlicht E., Barnard R. J., Grimditch G. K. Mechanism of insulin action on glucose transport in rat skeletal muscle. Am J Physiol. 1988 May;254(5 Pt 1):E633–E638. doi: 10.1152/ajpendo.1988.254.5.E633. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda N., Flanagan J. E., Kono T. Reassessment of insulin effects on the Vmax and Km values of hexose transport in isolated rat epididymal adipocytes. J Biol Chem. 1987 Feb 25;262(6):2737–2745. [PubMed] [Google Scholar]
- Yki-Järvinen H., Helve E., Koivisto V. A. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes. 1987 Aug;36(8):892–896. doi: 10.2337/diab.36.8.892. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Young A. A., Lamkin C., Foley J. E. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987 Jun;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L. THEORY OF THE USE OF ARTERIOVENOUS CONCENTRATION DIFFERENCES FOR MEASURING METABOLISM IN STEADY AND NON-STEADY STATES. J Clin Invest. 1961 Dec;40(12):2111–2125. doi: 10.1172/JCI104437. [DOI] [PMC free article] [PubMed] [Google Scholar]