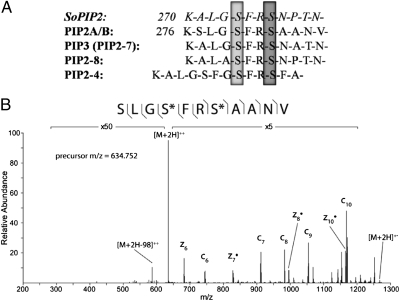
Fig. 2.
Decreased phosphorylation of aquaporins following ABA treatment. (A) Alignment of selected PIP family sequences. Dephosphorylation of S274 (light gray) in SoPIP2 was shown in structural studies to destabilize the “open” conformation of the protein, allowing closure of the water channel (20). This serine is conserved in all PIP family members quantified in this study and was shown to have decreased phosphorylation following ABA treatment. Additionally, the levels of phosphorylation of PIP2A/B in Arabidopsis at S280 (light gray) and S283 (dark gray) have been shown previously to change in response to other stressors (23, 24) and were found to be decreased in this study. (B) MS2 spectrum of PIP2A/B doubly phosphorylated peptide using ETD fragmentation in the control sample. Fragment ions detected are shown with the phosphopeptide sequence and are annotated in the spectrum. Both phosphorylation sites can be specifically assigned (*) by detection of flanking fragment ions around the phosphorylated residues.