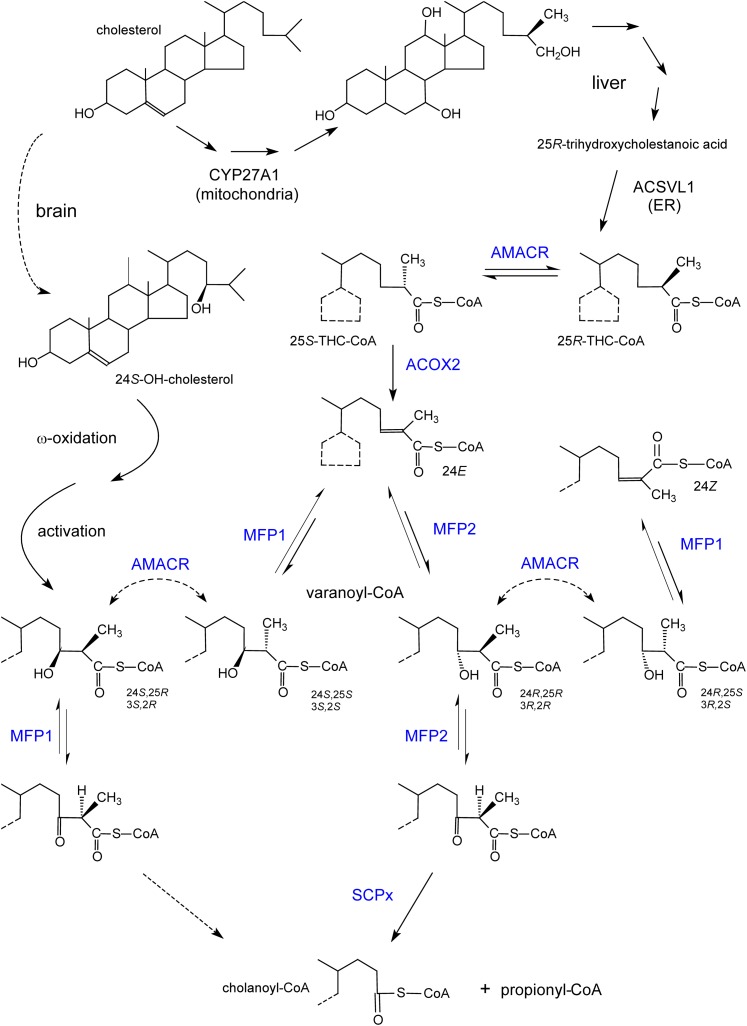
Fig. 5.
Formation of bile acids and stereochemistry of the involved enzymes. Shown at the top, starting from cholesterol through different steps (multiple arrows), mainly 25R-bile acid intermediates (e.g., trihydroxycholestanoic acid) are formed in humans due to the stereospecific reaction of 27-hydroxylase (CYP27A1). After activation in the ER, the CoA-ester is transported into peroxisomes and undergoes a chiral inversion to a 25S-isomer, which is desaturated by ACOX2. The enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase domains of MFP1 and MFP2 can produce or act on only one of the four possible 24-hydroxy-25-methyl-trihydroxycholestanoyl-CoA (also called varanoyl-CoA) stereoisomers. In contrast to the physiological pathway via MFP2 (with 24R-hydroxy-25R-methyl-cholestanoyl-CoA as intermediate), cholanoyl-CoA formation via MFP1 requires the assistance of racemase. A similar reasoning can be applied to pristanic acid (3R-hydroxy-2R-methyl-pristanoyl-CoA as the normal intermediate). The conversion of the 24R,25R-intermediate into 24Z-cholestenoyl-CoA, apparently a dead end product, via AMACR followed by a dehydratation by MFP1, has only been demonstrated in vitro. Shown at the left side, the ω-oxidized and activated product of the brain-derived cerebrosterol (24S-OH-cholesterol), given the 24S-hydroxy configuration, might be converted to primary bile acids via MFP1. Enzymes depicted in blue are peroxisomal.