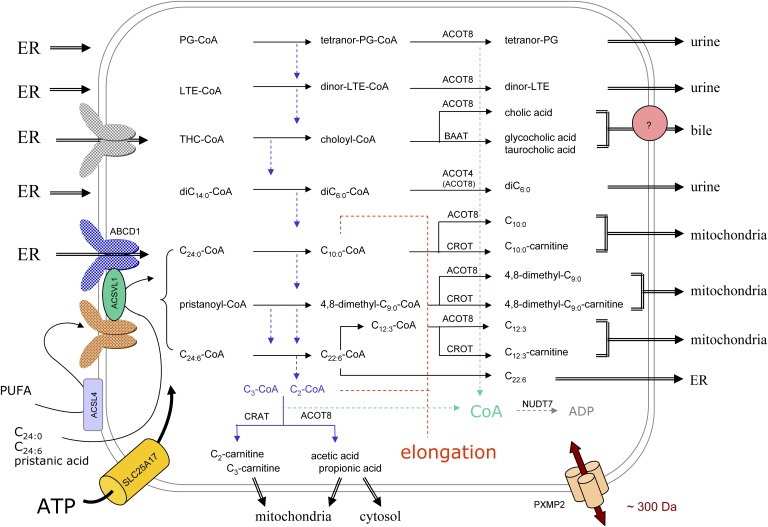
Fig. 6.
Organization of peroxisomal β-oxidation in relation to cellular metabolism. In this scheme, the subcellular origin of the major peroxisomal substrates (left side) and the fate of their degradation products (right side) are depicted. Due to the tissue-specific expression, not all depicted reactions will take place in peroxisomes of a particular cell type. The majority of substrates are activated in the ER (left side) and ABCD transporters, drawn in different colors, likely play a role in the uptake of the CoA-esters. Some FAs can be activated at the peroxisomal membrane or in the matrix, the required ATP entering via SLC25A17, but it is not known how these FAs cross the membrane. Depending on the presence of a 2-methyl group in the substrate, acetyl- or propionyl-CoA is produced (blue dashed arrows) that are normally shuttled to the mitochondria, as carnitine ester or free acid, but can be used for elongation (red dashed lines). The shortened substrates are generally processed in such manner that CoA is formed intra-peroxisomally, apparently a common theme during β-oxidation (green dashed arrows). This CoA can be reused by synthetases or thiolases or degraded by NUDT7. The β-oxidation end products can leave the organelle by passive diffusion, via the PXMP2 pore, or if larger than the pore diameter, via transporters. For bile acid conjugates (433–515 Da), there is biochemical evidence for a transporter (pink circle). Small solutes such as those involved in the conjugation or transferase reactions (glycine, taurine, carnitine) likely enter peroxisomes via the PXMP2 pore.