Abstract
In rat seminiferous tubules (ST), cells that contain polar and neutral lipids with long-chain polyenoic fatty acids (PUFA) and sphingomyelins (SM) and ceramides (Cer) with very long chain (VLC) PUFA of the n-6 series coexist. In this study, pachytene spermatocytes and round spermatids were isolated to determine how these lipids change during spermatogenesis. As the amount per cell of PUFA-rich glycerophospholipids (GPL) decreased with cell size, the 22:5/20:4 ratio increased with cell differentiation. The elovl2 and elovl5 genes, required for 22:5 formation, were expressed (mRNA) in both cell types. Residual bodies- particles with compacted organelles and materials discarded from late spermatids-concentrated cholesterol, 22:5-rich triacylglycerols, and GPL, including plasmalogens and phosphatidylserine. Species of SM and Cer with nonhydroxylated (n-) VLCPUFA (28:4, 30:5, and 32:5) predominated in pachytene spermatocytes, whereas species with the corresponding 2-hydroxy (2-OH) VLCPUFA prevailed in round spermatids. Thus, a dramatic increase in the 2-OH/n-VLCPUFA ratio in SM and Cer was a hallmark of differentiation. A substantial decrease of 2-OH SM occurred between spermatids and mature spermatozoa and 2-OH SM species were collected in residual bodies “en route” to Sertoli cells. Notably, spermatids and spermatozoa gained a significant amount of ceramides devoid of n-VLCPUFA but having 2-OH VLCPUFA as their main fatty acids.
Keywords: ceramides, elongases, glycerophospholipids, pachytene spermatocytes, spermatids, triacylglycerols, residual bodies, sphingomyelins, very long chain fatty acids
Mammalian spermatogenesis is an extraordinary cell transformation process. It includes asymmetric division of spermatogonia, rapid proliferation and differentiation into primary spermatocytes, a meiotic phase in which the genetic material in spermatocytes is recombined and segregated to produce haploid cells, a postmeiotic phase in which secondary spermatocytes develop into round spermatids, and differentiation of the latter into late spermatids and spermatozoa (1). During this last stage, known as spermiogenesis, spermatids undergo relevant cytological transformations, with changes in nuclear shape, chromatin condensation, formation of acrosome and flagellum, and disposal of surplus organelles and materials by production and release of membrane-enclosed remnants known as “residual bodies” (2, 3). These minute, densely packed particles are then engulfed by Sertoli cells (4).
The sphingomyelin (SM) (5) and ceramide (Cer) (6) of cells located in mammalian seminiferous tubules are rich in very long chain (C24 to C34) polyunsaturated fatty acids (VLCPUFA) of the n-6 or the n-3 series, the main ones being 28:4n-6 and 30:5n-6, followed by 32:5n-6 in the rat. These lipids belong to spermatogenic cells, as indicated by the facts that i) in the prepubertal rat testis, VLCPUFA-containing species of SM and Cer are not detected; ii) in conditions like cryptorchidism, administration of specific antitumoral drugs, and X-ray irradiation, which result in the selective death of spermatogenic cells from the testes of adult fertile rats, these species are lost (6); and iii) SM (7) and Cer (8) with VLCPUFA are quantitatively major components of mature spermatozoa in several species.
In addition to the “normal” (nonhydroxylated) VLCPUFA (n-VLCPUFA), the SM (9) and, more recently, the Cer (S. R. Zanetti, M. I. Aveldaño et al., unpublished observations) of rat testis and spermatozoa were shown to contain 2-hydroxylated versions of these unique fatty acids (2-OH VLCPUFA). In vivo exposure of the adult rat testis to a single dose of X rays damages germ cell precursors, leading to interruption of spermatogenesis. Those germ cells that survive irradiation are able to complete their differentiation and gradually leave the testis as spermatozoa, until only Sertoli cells remain in seminiferous tubules. This continuing germ cell depletion, taking a number of weeks, is concomitant to the exhaustion of SM and Cer species with both types of VLCPUFA (10). Species with n-VLCPUFA and with 2-OH VLCPUFA disappeared from the testis four and six weeks after irradiation, respectively, the former with the last spermatocytes, the latter with the last spermatids and spermatozoa. These in vivo transformations suggested an association between germ cell differentiation and SM and Cer species with each type of VLCPUFA. In the present study, rat pachytene spermatocytes and round spermatids were isolated to directly assess this possibility.
In isolated cells from rat (11) and mouse (12) testes, the proportion of 22:5n-6 increases, while that of 20:4n-6 decreases in glycerophospholipids (GPL) from spermatogonia to spermatids. Whereas the amount of 22:5n-6 in GPL progressively increases in the testis with spermatogenic cell growth and differentiation (13), it falls with the involution the mature organ undergoes as it gradually loses its germ cells as a long-term consequence of damaging stem spermatogonia after treatment with a single dose of X-ray irradiation (10). In the present study, the main GPL classes and subclasses that contribute to these differentiation-related changes were analyzed in pachytene spermatocytes, round spermatids, and residual bodies to contrast them as germ cell products with the VLCPUFA-containing SM and Cer.
Whether developing germ cells synthesize their own 22:5n-6 or depend on Sertoli cells for this matter has not been established. The biosynthesis of 22:5n-6 in cells has been shown to occur through a complex mechanism that requires i) the formation of 24:4n-6 in the endoplasmic reticulum; ii) transport of this product to peroxisomes; and iii) δ-6 desaturation to 24:5n-6, followed by one round of β-oxidation of the latter to 22:5n-6 (14). The two key elongation steps, namely 20:4n-6 → 22:4n-6 and 22:4n-6 → 24:4n-6 are catalyzed by the products of the Elovl2 and Elovl5 genes, respectively, which have been located in the human and mouse genomes and found to be actively expressed in the testis (15). In the present study, the mRNA produced from these genes was evaluated in rat pachytene spermatocytes and round spermatids.
In addition to membrane lipids, a group of intracellular neutral lipids is produced in cells located in rat seminiferous tubules that contain 22:5n-6 and VLCPUFA of diverse lengths: triacylglycerols (TAG), alkyl-diacylglycerols (ADG), and cholesterol esters (CE) (16). In vivo, after a single exposure to X rays, species of CE with 22:5n-6 steadily accumulate in the testis as it loses its germ cells, whereas 22:5n-6-rich TAG are virtually unchanged as long as spermatids continue to develop into spermatozoa (10). This observation suggested an association of CE with Sertoli cells and TAG with germ cells. By including in the present study the CE and TAG of pachytene spermatocytes, round spermatids, and residual bodies, the different cellular origin and, therefore, the different function of these two main neutral lipids are confirmed.
Thus, from a primary interest in the PUFA- and VLCPUFA-containing lipids of developing germ cells, investigation of the same lipids in residual bodies revealed several biologically relevant peculiarities of the spermatogenic cycle. The known function of these particles is to transport superfluous materials that are compacted and shed from differentiating spermatids, destined to be phagocytized and then utilized by Sertoli cells. The peculiarities of their PUFA- and VLCPUFA-rich lipids open new questions to solve in the dynamic picture of lipid involvement in germ cell differentiation. The inclusion of mature spermatozoa in this study further surprised us with additional differentiation-related changes in SM and Cer.
MATERIALS AND METHODS
Seminiferous tubules and cell fractions
Preparation of rat spermatogenic cells.
Sixty day-old Wistar rats were employed. The animals were sacrificed by cervical dislocation after a brief exposure to CO2 and used immediately. Seminiferous tubules were isolated from testes in a Krebs-Henseleit (KH) medium supplemented with 0.5 mM of CaCl2 and 10 mM of DL(+)-Lactate (KHs) and type I collagenase (0.5 mg/ml) and DNase (20 µg/ml) (KHs medium) to separate tubules from interstitial cells. Two major cellular fractions were separated in bulk from the tubules (17). Pachytene spermatocytes and round spermatids fractions were identified by size and the typical aspect of their nuclei stained with H33342 (18) (supplementary Fig. I). Owing to the large size of pachytene spermatocytes (cell diameter 17 ± 2 µm; range, 15–20 µm), their volume was calculated to be nearly 95% of the total cell volume in this fraction (88 ± 4% purity). The 12% cells contaminating this preparation were smaller cells (9.6% Sertoli cells; 1.8% round spermatids; and 0.6% leptotene and zygotene spermatocytes). The round spermatid fraction (cell diameter 11 ± 2 µm; range, 8–13 µm) had a calculated volume of round spermatids corresponding to approximately 98% of the total cell volume in this fraction (96 ± 2% purity). Contaminants were 0.8% late spermatids and 3.2% residual bodies. Additonally, a fraction enriched in residual bodies was obtained (size range, 3–5 µm, 77 ± 5% purity). The main contaminating cells and cell parts in this fraction were round spermatids (7%), condensed spermatids (13%), and sperm heads (13%).
The integrity of cell and residual body membranes was estimated by incubating suspensions containing 5 µM ethidium bromide and examining the cells under a fluorescence microscope. The cell membrane integrity was >95% under all conditions tested in this study. Protein content was determined in suspensions of cells and residual bodies using Bradford's reagents.
Rat epididymal spermatozoa
Cauda epididymi were excised, freed of their visible fat and blood vessels, and transferred to small dishes containing PBS. To allow release and diffusion of spermatozoa, a few incisions were made with a scalpel blade in the epididymal cauda, which were gently incubated in PBS under a 95% O2–5% CO2 atmosphere for 15 min at 34°C in a shaking water bath operated a 70 cycles per min. Sperm cells were recovered from supernatants by centrifugation at 600 g. They were then resuspended in 65% (w/v) sucrose at 4°C and purified in a discontinuous sucrose density gradient. As previously described (8), all media contained 2.5 mM EDTA to inhibit bivalent cation-dependent reactions, including the acrosomal reaction, which could affect spermatozoal lipids.
Lipid droplet detection
Rat testes were dissected, immersed in fixative, rinsed in PBS containing 30% sucrose overnight at 4°C for cryoprotection, and placed in a small amount of OCT (optimal cutting temperature) compound (Crioplast®, Biopack) on plastic stubs. The preparations were frozen, cut into 5–7 µm sections using a cryostat, and processed for Nile red staining as previously described (10). To stain lipid droplets in isolated cell fractions, suspensions were placed in small dishes with KHs medium and incubated for 5 min in the presence of Nile red. All samples were observed in a Nikon fluorescence microscope equipped with 40× NA 1.0 objective.
Elovl2 and Elovl5 expression
Total RNA was extracted from enriched fractions of pachytene spermatocytes and round spermatids using Trizol (Invitrogen, Carlsbad, CA). Equal amounts of RNA (2 µg) were reverse-transcribed with Ready-To-Go You-Prime First-Strand Beads kit (GE Healthcare, Piscataway, NJ) using oligo-dT as primers. For PCR, paired primers were designed for rat Elovl2 5′-CAG CAC ACA GGC ACC AGG-3′/ 5′-GAC TTC AGT GGC TCT CAC GG-3′ (568 base pair), rat Elovl5 5′-ACC ACC ATG CCA CTA TGC T-3′/ 5′-GTG TCC ATT GAC GGC AGT-3′ (407 base pair), and β-actin 5′-ATG GAT GAC GAT ATC GCT G-3′/ 5′-ATG AGG TAG TCT GTC AGG-3′ (569 base pair). Samples were denatured at 94°C for 5 min, followed by amplification rounds consisting of denaturing at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s for 30 cycles and 72°C for 10 min.
Lipid separation and analysis
After collecting germ cells, residual bodies and spermatozoa by centrifugation, lipid extracts were prepared and partitioned according to Bligh and Dyer (19). After lipid extraction, the organic solvents were evaporated under N2 and the samples dissolved in chloroform-methanol (2:1 v/v). Aliquots were taken for total lipid phosphorus, phospholipid composition, and total lipid fatty acid analyses.
For phospholipid class composition, aliquots of the extracts were spotted on HPTLC plates (Merck, Germany), along with commercial standards, and resolved with chloroform:methanol:acetic acid:water (50:37.5:3.5:2 by vol) (20). Phospholipids were located with iodine vapors; the silica gel containing the lipid spots was carefully transferred to tubes, and phosphorus was measured after digestion with perchloric acid (21).
Most of the rest of the lipid extracts was spotted on preparative TLC plates (500 µm, silica gel G) under N2, along with commercial standards (Sigma Chemical, MO). The neutral lipids were resolved in two steps. Chloroform:methanol:aqueous ammonia (90:10:2 by vol) was run up to the middle of the plates to separate the ceramides. The plates were removed, the solvents were evaporated, and a mixture of n-hexane:diethyl ether (80:20 by vol) was allowed to run up to near the top of the plates to resolve free and esterified cholesterol, ether-linked triglycerides, and triacylglycerols. The total polar lipid fraction was recovered from the origin of these plates and subjected to further separations and analyses. Aliquots were taken to study total GPL fatty acid composition. Part of the rest was used to preparatively isolate the GPL, on the one hand, and SM, on the other.
The GPL classes of germ cells and residual bodies were resolved using two-dimensional TLC (21). The major spots, choline GPL (CGP) and ethanolamine GPL (EGP), were recovered and further separated into their phosphatidyl- and plasmenyl- subclasses. Tubes with the eluted, dried GPC and EGP samples were briefly (1 min) agitated with a solution of 0.5 N HCl in acetonitrile, rapidly evaporating this solvent. The resulting lipids were immediately subjected to TLC (chloroform:methanol:water; 65:25:4 by vol) to separate 1) the fatty aldehydes running at the solvent front; 2) the small band of 2-acyl-GPL (formed from plasmalogens losing their fatty aldehydes at sn-1); and 3) a major fraction containing the most abundant 1,2-diacyl-GPL plus the minor 1-alkyl-2-acyl-GPL subclasses. The separated alkyl chains were not analyzed in the present samples of germ cell plasmalogens, but they were identified as fatty aldehydes by leaving room in the plates to run hexane:ether (95:5 by vol) up to the top of the plates and locating them with the aid of a commercial standard of 18:0 aldehyde. After elution, phosphorus and fatty acid analysis were performed on the polar moiety of the GPL.
In two dimensional TLC separations of phospholipids, SM was observed to separate neatly into two spots (SM1 and SM2). As shown in supplementary Fig. III, these two bands were made up of groups of molecular species that differed in the type of fatty acids amide-bound to sphingosine.
A large part of the polar lipid fraction recovered after isolating the neutral lipids was used to preparatively isolate the total sphingomyelin (SM) from the cell fractions under study by using chloroform:methanol:acetic acid:0.15 mol/l NaCl (50:25:8:2.5 by vol) as solvent.
After recovery of Cer and SM from the corresponding neutral and polar lipid separations, a mild alkali treatment was routinely performed to remove any potential lipid contaminant containing ester-bound fatty acids (6). After this alkaline treatment, SM and Cer were recovered again by TLC.
After TLC, all lipid bands were located under UV light after spraying with 2′,7′-dichlorofluorescein, and the zones containing the lipids were scraped into tubes for elution. This was performed by three successive extractions with chloroform:methanol:water (5:5:1 by vol), thoroughly mixing, centrifuging, collecting the solvents, and partitioning with 4 vol of water to recover the lipids in the chloroform phase. After elution, free cholesterol was measured using an enzymatic standard method.
Fatty acid analysis
The fatty acid composition of all lipid classes was determined by gas-chromatography (GC) after conversion of the eluted, dried lipids into fatty acid methyl esters (FAME). These were prepared by transesterification with 0.5N H2SO4 in anhydrous methanol under N2 (overnight at 45°C) in Teflon®-lined, screw-capped tubes (22). Before GC, FAME were routinely purified by TLC on silica gel G plates that had been prewashed with methanol:ethyl ether (75:25 by vol) and dried. Hexane:ether (95:5 by vol) was used to separate FAME, which were located with 2′,7′-dichlorofluorescein as described for other lipids. To elute FAME from the silica, this support was transferred to tubes and thoroughly agitated with water:methanol:hexane (1:1:1 by vol), followed by centrifugation to recover the upper hexane layer, repeating three times the hexane extraction, combining the upper phases, and drying under N2.
The methyl esters of n-VLCPUFA and 2-OH VLCPUFA derived from SM and Cer were separated by running hexane:ether (80:20 v/v) up to the middle of the plates, followed by hexane/ether (95:5 v/v) up to near the top, after methyl heneicosanoate and methyl 2-OH lignocerate had been added as internal standards (S. R. Zanetti, M. I. Aveldaño et al., unpublished observations).
The ordinary nonhydroxy FAME from all lipids, including n-VLCPUFA from SM and Cer, were directly analyzed by GC. The 2-OH FAME from SM and Cer were converted into O-trimethylsilyl (O-TMS) derivatives before GC by dissolving the samples in 50 µl hexane and adding 100 µl of a mixture of N,O-bis (trimethylsilyl)trifluoroacetamide (BSTFA) and 5% trimethylchlorosilane (TMCS) (Sigma-Aldrich, Fluka reagent). The samples were kept overnight in small conical silanized tubes with Teflon-lined caps at 45°C under N2 with the reactants. The latter were evaporated and the O-TMS ethers, taken up into hexane, were subjected to GC. The VLCPUFA of SM and Cer were identified by procedures and criteria described in detail in previous work, including mass spectrometry of major 2-OH VLCPUFA (10).
For fatty acid analyses, a Varian 3700 gas chromatograph equipped with two (2 m × 2 m) glass columns packed with 10% SP 2330 on Chromosorb WAW 100/120 (Supelco) was used. The column oven temperature was programmed from 150°C to 230°C at a rate of 5°C/min for (nonhydroxy) FAME of all lipids and from 180° to 230°C for the O-TMS of 2-OH FAME (S .R. Zanetti, M. I. Aveldaño et al., unpublished observations), and then kept at the upper temperature as required to elute the components with the longest chains. Injector and detector temperatures were set at 220°C and 230°C, respectively, and N2 (30 ml/min) was the carrier gas. The fatty acid peaks were detected with flame ionization detectors operated in the dual-differential mode and quantified using the software provided by a Varian Workstation.
Statistical analyses
One-way ANOVA was used to determine differences among mean values, which were compared using Tukey's multiple comparison test. All figures are mean values ± standard deviation, from four different samples obtained in independent isolations of seminiferous tubules, germ cells, and spermatozoa.
RESULTS
The amounts of protein, phospholipids (PL), free cholesterol, and other neutral lipids per cell (or particle) in the pachytene spermatocyte, round spermatid, and residual body preparations employed in this study are compared in Table 1. Although average cell size disparity was mostly responsible for these differences, the preparations showed interesting variations in main lipid concentrations and ratios. The PL/protein ratio (mg/mg) was higher in pachytene spermatocytes (0.25) than in round spermatids (0.19) and highest in residual bodies (0.40). Similarly, the free cholesterol/protein ratio (mg/mg) (× 10−1) was 0.21, 0.18, and 0.45, and the TAG/protein ratio (on the same basis) was 0.12, 0.11, and 0.62, respectively. Total PL, free cholesterol, and TAG were the main lipid classes in the three preparations, with a similar ratio among them in the two types of germ cells (87:7:4) but with a higher proportion of cholesterol and, notably, TAG in residual bodies (77:9:12), as estimated per mg of protein or per unit of cell volume. Other neutral lipids (diacylglycerols, cholesterol esters, triglycerides with an ether bond), represented not more than 2% of the total lipid in the three preparations, with residual bodies showing a significantly larger proportion of diacylglycerols than germ cells.
TABLE 1.
Main components of cellular fractions
| Pachythene Spermatocytes | Round Spermatids | Residual Bodies | |
|---|---|---|---|
| mg per 109 cells or particles | |||
| Proteins | 199 ± 22a | 110 ± 17b | 25 ± 4c |
| Phospholipids | 51 ± 3a | 21 ± 1b | 10 ± 0.5c |
| Free cholesterol | 4.2 ± 0.1a | 1.9 ± 0.1b | 1.2 ± 0.1c |
| Cholesterol esters | 0.15 ± 0.02a | 0.03 ± 0.01b | 0.02 ± 0.004b |
| Ether-linked triglycerides | 0.41 ± 0.03a | 0.08 ± 0.003b | 0.03 ± 0.004c |
| Diacylglycerols | 0.33 ± 0.02a | 0.20 ± 0.01b | 0.16 ± 0.01b |
| Triacylglycerols | 2.46 ± 0.24a | 1.25 ± 0.11b | 1.56 ± 0.08c |
| TAG/PL (mg/mg) | 0.05 ± 0.01a | 0.06 ± 0.01a | 0.16 ± 0.01b |
| Chol/PL (mol/mol) | 0.18 ± 0.01a | 0.20 ± 0.01a | 0.25 ± 0.002b |
The amounts of protein, phospholipids, free cholesterol, and other neutral lipids (mg) are compared among pachytene spermatocytes, round spermatids, and residual bodies. The content of cholesterol esters, triacylglycerols, diacylglycerols, and ether-linked triglycerides was estimated from their fatty acids. ANOVA and Tukey's multiple comparison test was used for statistical analysis. Chol, cholesterol (free); PL, phospholipid; TAG, triacylglycerol. Row values with different superscript letters (a,b,c) point to significantly different values (P < 0.05).
Phospholipid classes, subclasses, and main PUFA
The two cell types of this study had the same PL composition, with choline glycerophospholipid (CGP) and ethanolamine glycerophospholipid (EGP) being the main lipid classes (55% and 30% of the total PL, respectively), followed by nearly 4% each of SM, PI, and PS, and the remaining 2.5% the sum of diphosphatidylglycerol, lysophospholipids, and other minor lipids (Fig. 1). The total seminiferous tubules had more SM (7%) than each germ cell type or residual bodies, which may be attributed to Sertoli cells, as the latter contain twice as much SM as germ cells (23). One interesting peculiarity of residual bodies was that, compared with germ cells, they had more than twice the proportion of PS (8.5%).
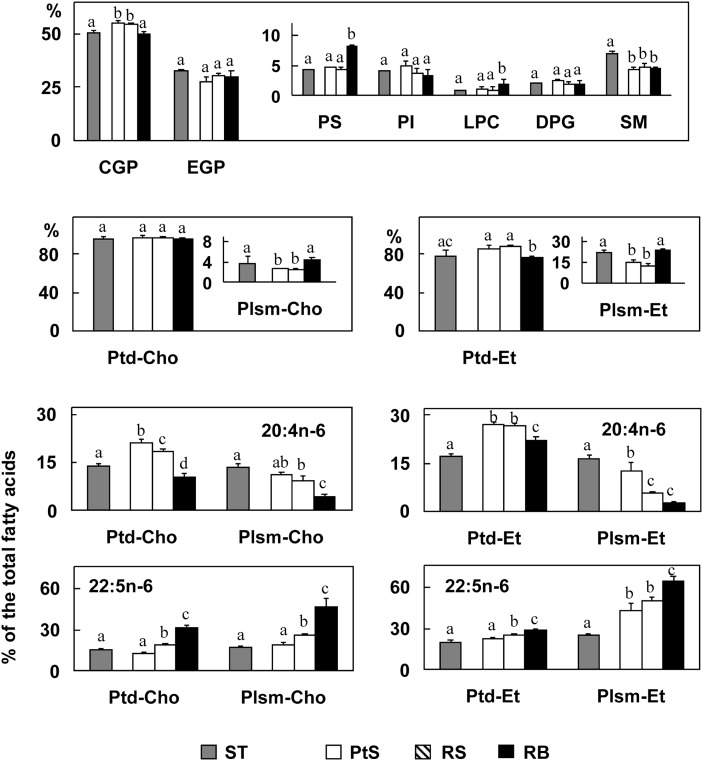
Fig. 1.
Upper and middle panel: Phospholipids of germ cells and residual bodies and their main PUFA. Pachytene spermatocytes (PtS), round spermatids (RS), and residual bodies (RB) (white, hatched, and black bars, respectively) are compared with each and with seminiferous tubules (ST, gray bars) from which they were obtained. Lower panel: Proportions of 20:4n-6 and 22:5n-6 in different CGP and EGP species (Ptd and Plsm) of germ cells and ST are compared. Values with different letters (a-d) are significantly different, P < 0.05. Abbreviations: CGP, choline glycerophospholipid; DPG, diphosphatidylglycerol; EGP, ethanolamine glycerophospholipid; LPC, lysophosphatidylcholine; PI, phosphatidylinositol; Plsm-Cho, plasmenylcholine (choline plasmalogen); Plsm-Et, plasmenylethanolamine (ethanolamine plasmalogen); PS, phosphatidylserine; SM, sphingomyelin.
Within CGP, phosphatidylcholine (1,2-diacyl-sn-glycero-3-phosphorylcholine) was the main subclass of germ cells with a small percentage (2%) of plasmenylcholine (the 1-alk-1′enyl,2-acyl-sn-glycero- subclass) (Fig. 1). Within EGP, phosphatidylethanolamine was the main subclass with a higher, but similar, proportion (13%) of plasmenyl-ethanolamine in the cells. Interestingly, residual bodies were almost twice as rich as germ cells in choline and ethanolamine plamalogens (4% and 24% of the total CGP and EGP, respectively).
The percentages with respect to the total fatty acids of 20:4n-6 and 22:5n-6 in the subclasses of CGP and EGP (Fig. 1) showed that both fatty acids were more abundant in the lipids of germ cells than in those of whole seminiferous tubules. Both were major PUFA of the major phosphatidylcholines and phosphatidylethanolamines of pachytene spermatocytes. The proportion of 20:4n-6 decreased while that of 22:5n-6 increased with the progression of differentiation to spermatids in the phosphatidyl- and especially in the plasmenyl- subclasses of both GPL. The 22:5n-6/20:4n-6 ratio in pachytene spermatocytes, round spermatids, and residual bodies, respectively, was 0.5, 1.0, and 4.0 in phosphatidylcholines; 2, 4, and 8 in plasmenylcholines; 0.8, 1.0, and 1.2 in phosphatidylethanolamines; and 4.0, 10, and 60 in plasmenylethanolamines, respectively. The residual body fraction displayed the largest plasmenyl/phosphatidyl subclass ratio in both GPL classes and the largest 22:5n-6/20:4 ratio in the four subclasses.
Elovl2 and Elovl5 expression in germ cells
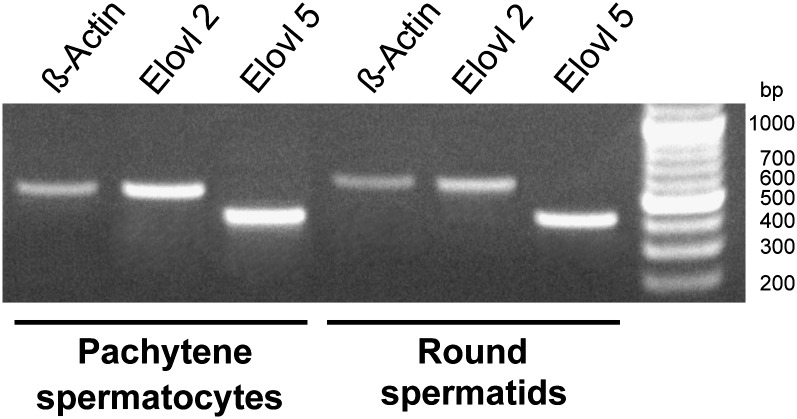
The results point out that 22:5n-6 is the major PUFA of rat testis because it abounds in the major GPL of both premeiotic and postmeiotic germ cells as well as residual bodies, collectively much more numerous than Sertoli cells. The biosynthesis of 22:5n-6 has been shown to require elongations of 20 and 22 carbon tetraenoic fatty acids to form 24:4n-6 in the endoplasmic reticulum; this product is then δ-6 desaturated to 24:5n-6, which is in turn β-oxidized to 22:5n-6, in peroxisomes (14). The (microsomal) elongation steps involved in such reactions are catalyzed by the products of the Elovl2 and Elovl5 genes. In rat pachytene spermatocytes and round spermatids, as isolated in the present study, the mRNA transcripts of both elongases were evidently expressed (Fig. 2).
Fig. 2.
Elovl mRNA expressions in pachytene spermatocytes and round spermatids. RNA was obtained from round spermatid- and pachytene spermatocyte-enriched fractions. After reverse transcription using Oligo-dTs, the cDNA was amplified using specific primers for rat Elovl5 (407 bp predicted size), Elovl2 (568 bp predicted size) and β-actin (569 predicted size). 100 bp DNA ladder are shown on the left lane. Results are representative of at least three separate experiments giving similar results. Abbreviation: Elovl; fatty acid elongase.
Neutral lipids and their fatty acids
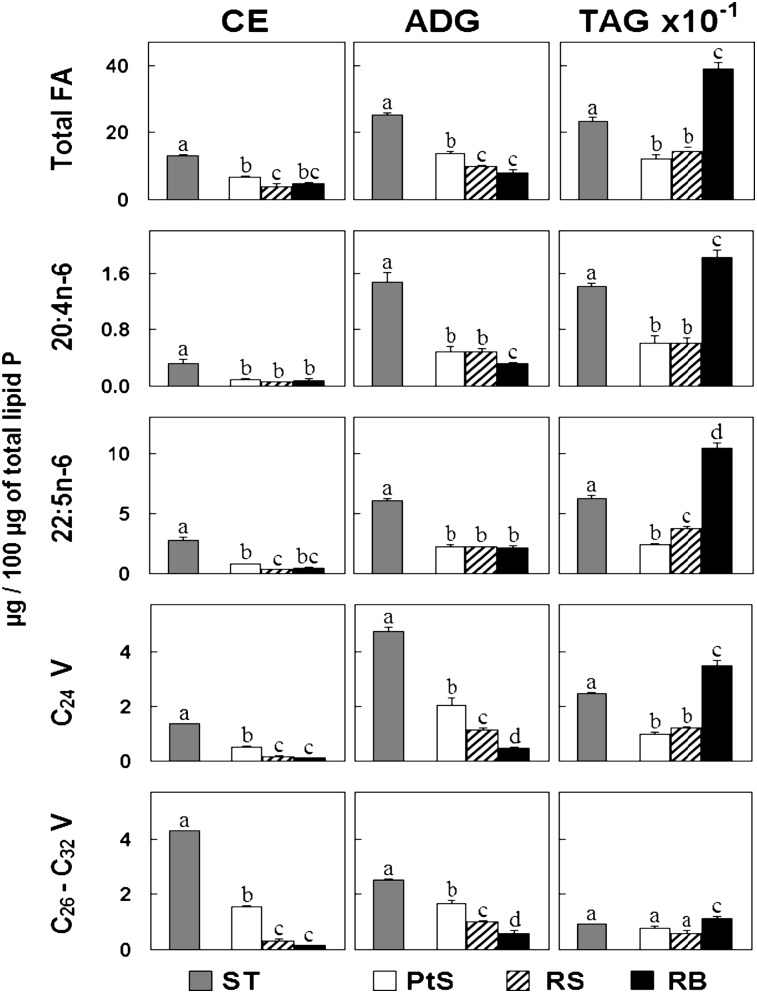
The three main neutral lipids present in rat seminiferous tubules—CE, ADG, and TAG—contained large amounts of 22:5n-6, low amounts of 20:4n-6, dissimilar proportions of C24 polyenes (24:4n-6 and 24:5n-6), and a collection of C26-C32 VLCPUFA (Fig. 3), the rest accounted for by ordinary saturated, monoenoic, and other PUFA (e.g., 18:2n-6). The individual PUFA constituents are shown in supplementary Fig. II. In CE, species with C26-C32 VLCPUFA predominated, followed by species with 22:5n-6. In ADG, the amount of 22:5n-6 was greater than the amount of C24 polyenes, and the amount of C24 polyenes was greater than the amount of C26-C32 polyenes. In TAG, there was a net predominance of 22:5n-6 over the other polyenes.
Fig. 3.
Concentrations of three neutral lipids on the basis of their fatty acids in germ cells and residual bodies. Pachytene spermatocytes (PtS), round spermatids (RS), and residual bodies (RB) (white, hatched, and black bars, respectively) are compared with each and with seminiferous tubules (ST, gray bars). The lipids were quantified by their fatty acids, and their concentrations are expressed on the basis of a fixed amount of lipid phosphorus (note the ten times larger scale for TAG concentration). “C24 V” refers to the sum of 24:4n-6 + 24:5n-6; “C26–C32 V” refers to the sum of all very long chain tetraenoic and pentaenoic fatty acids having this chain length range. Values with different letters (a-d) are significantly different, P < 0.05. Abbreviations: ADG, triglyceride with an ether bond; CE, cholesterol ester; TAG, triacylglycerol; V, very long chain polyenes.
As shown by their total and individual fatty acids (Fig. 3), the concentrations of CE and ADG were considerably higher in STs than in the two germ cell types isolated from STs and residual bodies, suggesting that these two neutral lipids may be mainly products of Sertoli cells. At least for CE, this interpretation agrees with the observation that, in vivo, lipid droplets and CE accumulate in these cells after the testis is deprived of germ cells by X-ray irradiation (10).
Regarding TAG, although their concentration was also higher in STs than in the two germ cell types, in this case, residual bodies (Fig. 3) were major contributors to the total TAG found in STs.
From pachytene spermatocytes to round spermatids to residual bodies, the concentration of species of TAG with 20:4n-6 was unchanged, whereas that of species with 22:5n-6 increased significantly. The 22:5n-6/20:4n-6 ratio increased in TAG in the same direction as in GPL. Thus, a considerable amount of fatty acids from germ cells, compacted into residual bodies in the form of TAG and membrane-bound GPL, is destined to be eventually incorporated into Sertoli cells.
Neutral lipid droplets
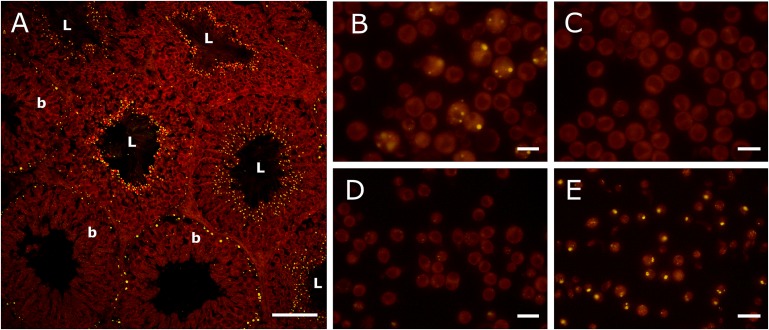
The use of Nile red as a fluorophore pointed to the existence of two populations of lipid inclusions in rat seminiferous ephitelium (Fig. 4). One was a collection of small lipid droplets facing the luminal duct in nearly 70% of the tubules. These lipid inclusions abounded at stages with elongated spermatids in differentiation to spermatozoa, and the fluorescent dye evidently marked the lipid-laden residual bodies. The other was a population of larger lipid droplets appearing in about 30% of the tubules, accumulating in Sertoli cells in cytoplasmic areas close to their nuclei and proximal to the basal membrane. In different sections of the seminiferous tubules, there were mostly one or the other type of lipid droplets, suggesting association with specific stages of the spermatogenic cycle.
Fig. 4.
Fluorescence microscopy of testicular lipid droplets, as revealed by Nile red. (A) Frozen section of a rat testis, showing the intense concentration of small lipid droplets near the lumen (L) of some seminiferous tubules and the more disperse and larger lipid droplets located near the basal (b) membrane of other tubules (bar = 100 µm). Fractions prepared from seminiferous tubules: Sertoli cells (B), pachytene spermatocytes (C), round spermatids (D), and residual bodies (E) (bar = 20 µm). Note the faint staining of the two germ cell fractions in contrast with the bright lipid droplets of Sertoli cells and especially of residual bodies.
When Nile red was employed in isolated germ cells, no lipid droplets were detected in pachytene spermatocytes or round spermatids (Fig. 4). These cells stained faintly with the fluorophore, in contrast to the intense dot-shaped fluorescence displayed by residual bodies. Because the latter were extremely rich in TAG whereas they had barely detectable amounts of CE and ADG (Fig. 3), it is apparent that TAG are the main components of the neutral lipid inclusions present in these particles.
In fractions that contained Sertoli cells, large and small lipid droplets were observed, in some cases coexisting (Fig. 4). One may infer that the larger and the smaller type of droplets may contain mostly CE and TAG, respectively. The larger ones were observed concomitantly with the highest concentrations of CE in seminiferous tubules having only Sertoli cells (germ-cell deprived) from rat testis (10). Considering their size, the smaller ones may represent (TAG-rich) residual bodies recently endocytosed by Sertoli cells.
Germ cell sphingomyelins, ceramides, and their VLCPUFA
As observed in relative terms from the phospholipid composition (Fig. 1), the concentration of SM was similar in pachytene spermatocytes, round spermatids, and residual bodies (Fig. 5), but it was significantly higher in seminiferous tubules. This is attributable to the fact that Sertoli cells are about four times as rich in SM as germ cells. In clear contrast with SM, the bulk of Cer present in seminiferous tubules was mostly contributed by germ cells. The Cer content and the Cer/SM ratio increased in the order pachytene spermatocytes → round spermatids.
Fig. 5.
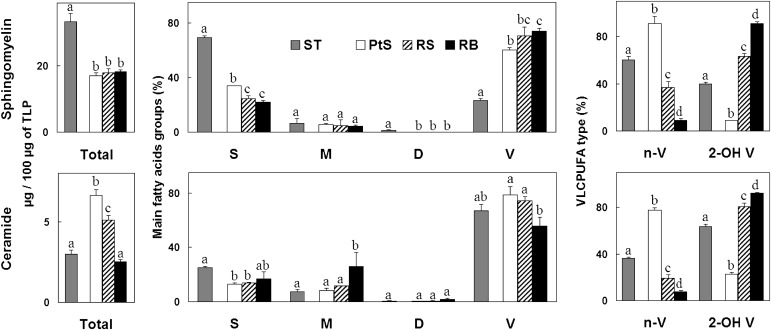
Sphingomyelin and ceramide fatty acids in germ cells. Pachytene spermatocytes (PtS), round spermatids (RS), and residual bodies (RB) (white, hatched, and black bars, respectively) are compared with each and with the seminiferous tubules (ST, gray bars) from which they were derived. The lipids were quantified by their fatty acids and expressed as µg per a fixed amount of total lipid phosphorus (TLP). Left panels: amounts of total fatty acids; middle panels, amounts of main fatty acids, grouped into saturated (S), monoenoic (M), dienoic (D), and total VLCPUFA (V); right panels: percentage (%) contribution of nonhydroxy (n-V) and 2-hydroxy (2-OH V) VLCPUFA to the total fatty acids present in the VLCPUFA (V) fraction. Values with different letters (a-d) are significantly different, P < 0.05.
In seminiferous tubule SM, species with saturated fatty acids predominated (Fig. 5), revealing a characteristic of Sertoli cell SM, followed by species with VLCPUFA, mostly contributed by germ cells. Thus, in pachytene spermatocytes and round spermatids, SM species with VLCPUFA strongly predominated over the rest (65% and 70% of the SM fatty acids, respectively). The proportion of species of Cer with VLCPUFA was even higher (about 80% of the Cer fatty acids in both germ cell types).
The most dramatic change associated with germ cell differentiation on membrane-associated lipids was observed in SM and Cer VLCPUFA (Fig. 5). Species with n-VLCPUFA predominated extremely in pachytene spermatocytes (90% and 80% of the total VLCPUFA of SM and Cer, respectively). Their proportion was several-fold smaller in round spermatids and barely detectable in residual bodies. Conversely, while minor in the SM and Cer of pachytene spermatocytes, species with 2-OH VLCPUFA prevailed in round spermatids (65% and 80% of the total VLCPUFA of SM and Cer, respectively). This trend continued with differentiation, as inferred from the fact that 2-OH VLCPUFA were virtually the only VLCPUFA present in the SM of residual bodies.
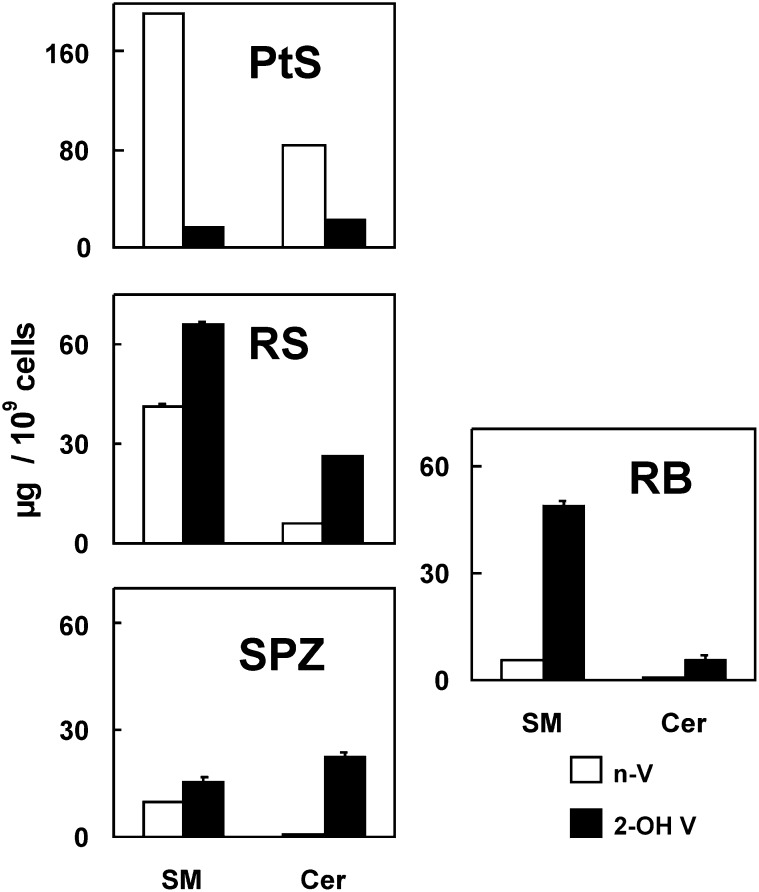
Data in Fig. 5 and Fig. 6 indicate that the 2-OH VLCPUFA/n-VLCPUFA ratio increased several fold in SM and Cer with germ cell differentiation. In addition, the value attained for this ratio in the SM of round spermatids was virtually the same as that present in the SM of spermatozoa isolated from the epididymal cauda.
Fig. 6.
Content of n-VLCPUFA (n-V, white bars) and 2-OH VLCPUFA (2-OH V, black bars) in SM and Cer of pachytene spermatocytes (PtS), round spermatids (RS), and residual bodies (RB) in comparison with epididymal spermatozoa (SPZ). With the progression of germ cell differentiation, the content of species of SM and Cer with both types of VLCPUFA per cell decreased (as did size), and the 2-OH VLCPUFA/N-VLCPUFA ratio increased several fold in both lipids. From RS to SPZ there was a loss of 2-OH SM that was mostly accounted for by 2-OH SM recovered in RB. These particles transported abundant SM but little Cer to Sertoli cells. Notably, differentiation entailed an increase of 2-OH VLCPUFA-rich ceramides as normal components of RS and Spz. Abbreviations: Cer, ceramide; SM, sphingomyelin.
An interesting quantitative aspect was that the amount per cell of n- and 2-OH VLCPUFA-containing species in SM decreased roughly 4-fold in the progression between round spermatids and spermatozoa (Fig. 6). As one spermatid gives rise to one spermatozoa, this change in SM fatty acids occurred concomitantly with the relevant changes in shape and size the gametes undergo during the last phases of spermiogenesis, which entail an intense remodeling of their membranes, ending up with the concentration and expulsion of no longer necessary materials in the form of residual bodies. The amount of 2-OH VLCPUFA-containing SM species per residual body particle roughly accounted for the difference in content between the amount per cell present in round spermatid and spermatozoa (Fig. 6).
In contrast to the described reduction per cell in the content of SM, mature spermatozoa retained a similar concentration of Cer with 2-OH VLCPUFA as was in their round spermatid predecessors. Consistently, virtually no Cer was disposed of in residual bodies.
It must be pointed out that in this study spermatozoa were isolated from cauda epididymi in the presence of a bivalent ion chelator to minimize the possibility that part of their SM would hydrolyze to Cer during their preparation (8). The gametes did thus contain a high endogenous amount of Cer with 2-OH VLCPUFA.
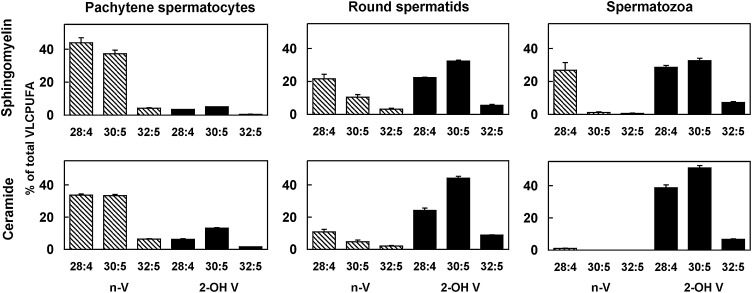
Figure 7 depicts how the described changes affect the main individual species with VLCPUFA of SM and Cer. The three major n-VLCPUFA of both lipids, 28:4n-6, 30:5n-6, and 32:5n-6, were highest in pachytene spermatocytes and tended to decrease almost proportionally to the increased proportions of 2-OH 28:4n-6, 2-OH 30:5n-6, and 2-OH 32:4n-6 with differentiation to round spermatids. Eventually, virtually no species of Cer with n-VLCPUFA remained in mature spermatozoa, which had SM species with 28:4n-6 as the main, and virtually the only, n-VLCPUFA (Fig. 7). In contrast, species with 2-OH VLCPUFA clearly predominated in both sperm lipids but remarkably in Cer (Fig. 6), with 2-OH 30:5n-6, 2-OH 28:4n-6, and 2-OH 32:5n-6 being the main sperm Cer fatty acids (Fig. 7).
Fig. 7.
SM and Cer main individual VLCPUFA of pachytene spermatocytes, round spermatids, and epididymal spermatozoa. n-V (hatched bars) and 2-OH V (black bars). Differentiation is shown to entail a notable increase in the proportion of SM and Cer species with 2-OH 28:4n-6 and especially 2-OH 30:5n-6 at the expense of 28:4n-6 and 30:5n-6. Notably, while sperm SM retains its 28:4n-6, sperm ceramides with “normal” VLCPUFA are eliminated with differentiation to spermatozoa. 2-OH V, 2-OH VLCPUFA; n-V, n-VLCPUFA.
DISCUSSION
The presented results show that, concomitantly with cell size reduction and increased number associated with spermatogenesis, germ cell differentiation implies a reduction in the amount per cell of membrane phospholipids, with a marked increase in the proportion of 22:5n-6 in GPL and of 2-OH VLCPUFA in SM. The virtually exclusive presence of n-VLCPUFA in the SM and Cer of pachytene spermatocytes and the net preponderance of species with 2-OH VLCPUFA in spermatids and spermatozoa pinpoint a novel hallmark of germ cell differentiation, even more radical than the increase of 22:5n-6 at the expense of 20:4n-6 observed in GPL. Several biologically relevant peculiarities in the lipids of residual bodies were disclosed: a higher proportion than germ cells of cholesterol; 22:5n-6-rich GPL; certain phospholipid classes, like phosphatidylserine and ethanolamine plasmalogens; an over-abundance of TAG as the only neutral lipid class present in their lipid inclusions; and a predominance of species with 2-OH VLCPUFA in their SM. Purified spermatozoa from the epididymis exhibited further cell differentiation-related changes, such as the presence of SM with both n-VLCPUFA and 2-OH VLCPUFA and abundant ceramides with only 2-OH VLCPUFA. Because residual bodies are engulfed by Sertoli cells as they are formed from condensing spermatids during spermiogenesis, the present results, showing that these tiny but numerous particles carry with them a lot of 22:5n-6-rich GPL and TAG, as well as 2-OH VLCPUFA-rich SM species, call attention to the fact that the next steps in the metabolism of these lipids and fatty acids must take place in Sertoli cells.
Germ cell membrane glycerophospholipids
As germ cells differentiate from the premeiotic pachytene spermatocytes to the postmeiotic round spermatids, the number of cells increases while their size (volume) decreases, with the amount per cell of total phospholipids decreasing in this maturational passage. In this process, part of the membrane phospholipids of the smaller daughter cells are “inherited” from their larger predecessors. Most of the de novo lipid biosynthetic activity probably occurs during the several days the cells stay as pachytene spermatocytes. In addition, a dynamic replacement of lipid molecules, or their parts, probably occurs with differentiation. The increased proportions of plasmalogens in spermatids with respect to spermatocytes may be due to these phospholipids decreasing less than others with differentiation. The increased proportion of 22:5n-6 in all GPL in the same direction may be contributed by spermatids synthesizing an extra amount of 22:5n-6 (from 20:4n-6) while conserving part of the original 22:5n-6. As differentiation progresses, GPL may be actively modified at their sn-2 positions by acyltransferases that introduce newly synthesized 22:5n-6. These enzymes are ubiquitous in tissues; their activity in rat pachytene spermatocytes and round spermatids has been revealed by the net incorporation of [14C]labeled C20-C22 PUFA these cells display, mostly into choline and ethanolamine GPL (24–26). The differentiation-related changes in main GPL species profiles may be expected to have an impact on the functional properties of germ cell membranes whose purpose is so far unknown.
It has been known for decades that plasmalogens are important, in some cases being the main, lipid constituents of spermatozoa. The enzymes that initiate the synthesis of ether-linked lipids—dihydroxyacetonephosphate acyltransferase (GNPAT) and alkyl-dihydroxyacetonephosphate synthase—are known to be located in the peroxisomal membrane. In the testis, the presence of peroxisomes has been demonstrated in somatic as well as in all germ cell types, except mature spermatozoa (27). Germ cells present marked differences in structure, abundance, and localization of peroxisomes, with a strong heterogeneity in peroxisomal protein type and content, in accordance to the multiple biosynthetic and catabolic functions located to these organelles. In mice with a knockout in the Gnpat gene, a spermatogenic arrest ensues, with apoptosis of pachytene spermatocytes and complete absence of elongated spermatids and mature spermatozoa (28), emphasizing the importance of ether-linked lipids for spermatogenesis.
Because residual bodies are formed from a portion of the membrane that buds from condensing spermatids that are ready to be released as spermatozoa, the significantly higher proportion of 22:5n-6-rich phospholipids, including plasmalogens, found in these remnant particles may reflect a general characteristic of the membranes of these late spermatids. Alternatively, it may be speculated that a clustering of specific phospholipids and free cholesterol may occur in the particular area of these spermatids where residual bodies are formed, favoring their detachment from ready-to-emerge spermatozoa.
The present observation that residual bodies are far richer in phosphatidylserine (PS) than are germ cells is a biologically relevant fact. Sertoli cells phagocytize apoptotic bodies from germ cells (hundreds of these cells normally die daily by apoptosis in the course of spermatogenesis) by recognizing, through their scavenger receptor type I (SR-I), the PS located on the surface of apoptotic fragments (29). The SR-I is expressed on both the lateral and the apical surfaces of Sertoli cells, such expression changing with the phases of the spermatogenic cycle (30). Sertoli cells could thus use the same mechanism for phagocytizing PS-exposing, germ cell-derived apoptotic bodies and residual bodies.
Regarding the specific and major PUFA of the major rat germ cell GPL, we showed that isolated pachytene spermatocytes and round spermatids expressed the Elovl2 and Elovl5 genes. This finding supports the notion that germ cells may be capable of synthesizing their quantitatively major PUFA, 22:5n-6, as concluded in previous work (25, 26) showing that both germ cell types are able to transform [1-14C]20:4, added as precursor, into labeled 22:4, 24:4, 22:5, and 24:5. Further work is still needed to reconcile this possibility with the fact that isolated germ cells have a low δ-6-desaturase activity (26) and mRNA expression (31) compared with isolated Sertoli cells. Considering that germ cells are far richer in 22:5n-6 than Sertoli cells, it was suggested (26, 31) that the latter could biosynthesize 22:5n-6 and supply it to germ cells. Although this possibility is attractive in view of the well-known structural and metabolic supporting roles Sertoli cells play in sustaining germ cell development, our results allow us to see “the other side of the coin.” Whereas transport of 22:5n-6 from Sertoli cells to germ cells remains to be demonstrated, Sertoli cells certainly receive abundant amounts of preformed fatty acids from spermatogenic cells in the form of 22:5-rich lipids enclosed in apoptotic and residual bodies. Thus, spermatogenic cells may be devoted to mostly anabolic tasks, generating at their own pace lipids with PUFA and VLCPUFA destined to become sperm components, whereas Sertoli cells may be busy most of the time with catabolic tasks regarding this type of lipid.
Germ cell-neutral lipids
An important result of the present study was the identification of the cellular origin and, thus, the likely different function of CE and TAG, two neutral lipid classes present in rat seminiferous tubules. With the aid of Nile red, residual bodies were detectable as numerous luminally located, brightly fluorescent, uniformly-sized minute dots within seminiferous tubules, precisely at stages at which fully developed spermatids evolve into spermatozoa. TAG were intensely concentrated in such structures and, therefore, were produced in the last stages of spermiogenesis by the most differentiated members of the spermatogenic lineage. Residual bodies contained TAG as virtually their only neutral lipid but no CE. Thus, TAG were primarily germ cell products, whereas the CE that abound in seminiferous tubules were not, supporting the previous conclusion, derived from germ cell depletion studies in vivo (10), that CE are mostly Sertoli cell products.
The fact that the concentration of TAG progressively increased as that of GPL did not change from pachytene spermatocytes to round spermatids to residual bodies, suggests that it is in the last steps of spermiogenesis that TAG synthesis is most stimulated, with high specificity for the esterification of C22-24 PUFA. Regarding TAG synthesis in germ cells, previous work in cultures of mouse-derived germ cells showed that (i) [14C]20:4n-6 can be elongated and desaturated (to 22:4, 22:5, 24:4, and 24:5) and (ii) pachytene spermatocytes incorporate lower percentages and absolute amounts of radioactivity into their TAG fatty acids derived from 20:4 by elongation-desaturation (22:4, 24:4) than do spermatids (round and condensing types) (25), in agreement with our observations. Interestingly, distributions of mass and radioactivity in these TAG showed that 22 or 24 carbon PUFA were incorporated with a high degree of specificity at the sn-3 position.
Accumulating spare fatty acids in the form of TAG is a universal room-saving strategy for storing these energy-rich substrates in cells. Part of the TAG shown here to be confined into residual bodies may represent a form of disposal of no longer needed GPL from the progressively smaller elongating-condensing spermatids. Once surplus membranes containing 22:5n-6-rich GPL are incorporated into the residual bodies in formation in such spermatids, removal of GPL phosphoryl base may produce diacylglycerols, while part of their ester-bound fatty acids may become free fatty acids; after further conversion of the former into the corresponding acyl-CoA derivatives, these products may easily combine, in part perhaps within the residual bodies themselves, to produce 22:5-rich TAG.
Because Sertoli cells are known to phagocytize residual bodies almost at the same rate as the latter are shed from condensing spermatids during spermiogenesis, the next steps in the metabolism of the (lipid and nonlipid) materials these particles transport is one among many metabolic “responsibilities” of Sertoli cells. The present results call attention on the fact that these particles transport a lot of 22:5n-6-rich GPL and TAG, as well as 2-OH VLCPUFA-rich species of SM, from developing germ cells to Sertoli cells.
After endocytosis, Sertoli cells may be expected to perform diverse forms of metabolic processing of each of the mentioned lipids before disposal and/or reutilization of the fatty acids. Sertoli cell lysosomes are good candidates for a first step of hydrolysis of the membrane-associated phospholipids incorporated with residual bodies. In the case of TAG, a form of hormone-sensitive lipase (HSL) has been detected by its immunreactivity in the luminally-facing part of the Sertoli cell cytoplasm, precisely at stages in which residual bodies are phagocytosed (32). This HSL could remove the fatty acids from the ingested TAG, allowing their FA to be immediately converted to CoA esters and utilized in these cells.
Taking into account that residual bodies also carry into Sertoli cells abundant GPL as potential sources of free fatty acids as well as of free cholesterol, both of which are toxic to cells, one may envisage these cells playing an important role in forming CE (in their endoplasmic reticulum) at spermatogenic stages at which they are loaded with these lipid materials, collecting these CE temporarily in the form of cytoplasmic lipid droplets. This could account for the 22:5n-6- and VLCPUFA-rich CE shown in this study to be abundant in seminiferous tubules, scanty in germ cells, and negligible in residual bodies.
SM and Cer
The present study shows that the type of species that contain VLCPUFA in germ cells changes dramatically with their differentiation. From SM and Cer species with nonhydroxylated VLCPUFA predominating in pachytene spermatocytes, species with 2-OH VLCPUFA prevailed in round spermatids. This is consistent with previous results in vivo, showing that after a single exposure of the rat testis to X-rays, in which depletion of spermatocytes precedes in two weeks that of spermatids and spermatozoa, SM and Cer species with n-VLCPUFA disappear from the testis two weeks before species with 2-OH VLCPUFA (10).
Further differences between round spermatids and fully mature spermatozoa let us see that a significant amount per cell of SM species with 2-OH VLCPUFA is shed from developing spermatids and is recovered in the form of an almost equivalent amount per particle of the same type of SM species in residual bodies. This suggests that these species are made in excess of the amount required in the final gametes. Concurrently, in round spermatids, an important amount per cell of endogenous Cer (on a mole basis, larger than the total SM) is formed that remains high in mature spermatozoa but is negligible in residual bodies. This suggests that such Cer are produced early in postmeiotic cells and become components of the mature gametes. Further research is needed to establish the role of these unique ceramides in sperm functions.
Regarding fatty acids, the selective decrease in the n-VLCPUFA/2-OH VLCPUFA ratio with differentiation is reflected in the final product of this process, spermatozoa, in which 28:4n-6 remains as virtually the only n-VLCPUFA of SM, whereas 2-OH VLCPUFA not only predominate in SM but become the almost exclusive type of VLCPUFA of Cer.
Of the six ceramide synthase (CerS) isoforms expressed in rat tissues, CerS3 is almost exclusively expressed in the testis (33). In the mouse testis, CerS3 is almost exclusively expressed in germ cells (34), Cer3 expression increasing concomitantly with the appearance of the first spermatocytes during postnatal development. This enzyme is thus responsible for the de novo formation of the ceramides of pachytene spermatocytes, the present results showing that it chiefly uses n-VLCPUFA as fatty acid substrates.
The 2-hydroxylation of n-VLCPUFA that results in the major 2-OH VLCPUFA of round spermatid Cer and SM requires fatty acid 2-hydroxylase activity (FA2H). In mice, the FA2H gene is highly expressed in brain, in accordance with the fact that myelin galactosyl sphingolipids are rich in 2-hydroxy fatty acids, but its expression is also considerable in testis (35). The present results suggest that such expression could be concentrated in spermatids, rich in SM and Cer with 2-OH VLCPUFA. In analogy with skin, where FA2H expression and activity increase with differentiation of keratinocytes (36), in the rat testis, SM and Cer fatty acid hydroxylation increases with differentiation of germ cells, as suggested by its lipid products. The mechanism of SM and Cer fatty acid hydroxylation remains to be established.
The last steps of the biosynthesis of SM require a SM synthase. SM synthase 2 (SMS2) is known to be the activity at the plasma membrane (37, 38) that catalyzes the conversion of (golgi-derived) Cer into SM (Cer + phosphatidylcholine → SM + diacylglycerol). The present results suggest that, in pachytene spermatocytes, ceramides with n-VLCPUFA may be substrates to form SM with the corresponding type of fatty acids. What happens in round spermatids is probably more complex, first, because a fatty acid 2-hydroxylation step is involved, and second, because the high Cer/SM ratio in these cells suggests that the formation of an excess of Cer may be required for an as-yet-unknown function in spermatozoa. Thus in these cells, the possibilities exist that (i) 2-OH VLCPUFA-containing Cer could be synthesized by CerS3 only as a final product; (ii) SMS2 could catalyze the formation of 2-OH VLCPUFA-containing SM from part of these Cer; and (iii) 2-OH VLCPUFA-containing SM could be in part hydrolyzed to the corresponding Cer by a form of sphingomyelinase.
In the seminiferous epithelium of rat testis, stage-specific expression of a form of SMS2 was localized to late round spermatids and early elongating spermatids and not present in late elongated spermatids or Sertoli cells (39). This enzyme was suggested to play a role in acrosome formation and the plasma membrane restructuring that occurs between late round spermatids and early elongating spermatids. This could be the SMS2 of round spermatids producing the 2-OH VLCPUFA-rich SM that at later developmental phases concentrate in residual bodies and spermatozoa.
The fact that spermatozoa endogenously carry from their origin high levels of Cer specifically containing 2-OH VLCPUFA is a striking finding of this study. The generation of such Cer from SM as precursor requires either (i) a form of SMase that is specific for 2-OH VLCPUFA-containing species of SM, or (ii) an ordinary SMase that expresses itself in a cell-specific manner in spermatids. Mice deficient in acid SMase in their lysosomes (a model of the human Niemann-Pick disease), accumulate SM in their tissues, including testis (mostly as lipid inclusions in Sertoli cells), and produce spermatozoa that develop an abnormal motility (40–42). The latter of these defects correlated with an excess of surface area in the midpiece of the sperm tail in late spermatids and spermatozoa, with the mature gametes showing a rigid bending of their tails that preclude normal motility. Taken together with the present results showing that (normally motile) rodent spermatozoa endogenously contain a significant amount of Cer with specific fatty acids, one may conclude that the sperm defect in acid SMase-deficient mice may have been that their spermatids were unable to produce the required Cer from their own SM during spermiogenesis.
The finding that 2-OH VLCPUFA-rich species of SM localize in residual bodies found no antecedent in the literature. One of the many questions that arise from this fact is what the biophysical or biochemical basis may be for the segregation of such species in residual bodies. One could speculate that a surplus of these SM concentrate on the specific surface areas of spermatids where residual bodies “sprout.” As these structures contained a relatively high amount of cholesterol compared with germ cells, a form of “lateral separation” of these SM species into cholesterol-rich domains (rafts) on the spermatid surface could be involved.
Once residual bodies are phagocytized by Sertoli cells, these cells may be expected to metabolize the SM thus incorporated. A first obvious step is to hydrolyze this lipid to Cer in Sertoli cell lysosomes. That this occurs through an acid SMase is evident from the accumulation of SM and abnormal lipid inclusions (probably SM-laden lysosomes) observed in Sertoli cells of mice deficient in this enzyme (42). Provided an acid ceramidase activity follows, the next question that arises is what Sertoli cells do with the resulting 2-OH VLCPUFA. They are probably rapidly converted into acyl-CoA derivatives as they are released from lysosomes and directed to other subcellular organelles for further utilization.
Because biosynthesizing 2-OH VLCPUFA is energetically quite costly, it is tempting to speculate that it would be convenient if Sertoli cells spared these fatty acids from oxidation and had a strategy of recycling, making these fatty acids available to their endlessly developing fostered neighbor cells. However, this possibility is challenged by the fact that transport of any form of lipid from Sertoli cells to germ cells remains to be demonstrated and that Sertoli cells contain peroxisomes (27) with active peroxisomal enzymes (43) where acyl-CoA esters of 2-OH VLCPUFA could be efficiently α-oxidized and then chain-shortened. These products could then be β-oxidized as, in contrast to germ cells, Sertoli cells contain all of the enzymes of the mitochondrial fatty acid β-oxidation system (44). Moreover, the expression of long-chain acyl-CoA dehydrogenase, a key enzyme in the β-oxidation of fatty acids, including PUFA, as well as the production of ATP, are both much lower in any testicular cell than in Sertoli cells, both increasing markedly in the latter after phagocytosis of apoptotic bodies from germ cells (45). The mass of spermatid-derived long-chain and very-long-chain fatty acids incorporated in Sertoli cells after phagocytosis of lipid-laden residual bodies could have the same fate: a source of the large amount of energy that supporting spermatogenesis imposes on these cells. The present results, showing that these remnant particles contain SM with only 2-OH VLCPUFA, may inspire future research to discern the metabolic fate of these molecular species.
Supplementary Material
Footnotes
Abbreviations:
- ADG
- triglyceride with an ether bond
- CE
- cholesterol ester
- Cer
- ceramide
- CGP
- choline glycerophospholipid
- DPG
- diphosphatidylglycerol
- EGP
- ethanolamine glycerophospholipid
- Elovl
- fatty acid elongase
- GC
- gas chromatography
- GPL
- glycerophospholipid
- LPC
- lysophosphatidylcholine
- PI
- phosphatidylinositol
- PL
- phospholipid
- PS
- phosphatidylserine
- PtS
- pachytene spermatocyte
- RB
- residual body
- RS
- round spermatid
- SM
- sphingomyelin
- SPZ
- spermatozoa
- ST
- seminiferous tubule
- TAG
- triacylglycerol
- VLCPUFA
- very long-chain polyunsaturated fatty acid. Please note that n- and 2-OH are used as prefixes to denote nonhydroxy and 2-hydroxy VLCPUFA, respectively.
This work was supported by funds from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción de la Ciencia y la Tecnología (ANPCyT), and Universidad Nacional del Sur (UNS), Argentina, and by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Russell L., Frank B. 1978. Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat. Rec. 190: 79–97. [DOI] [PubMed] [Google Scholar]
- 2.Lalli M., Clermont Y. 1981. Structural changes of the head components of the rat spermatid during late spermiogenesis. Am. J. Anat. 160: 419–434. [DOI] [PubMed] [Google Scholar]
- 3.Sprando R. L., Russell L. D. 1987. Comparative study of cytoplasmic elimination in spermatids of selected mammalian species. Am. J. Anat. 178: 72–80. [DOI] [PubMed] [Google Scholar]
- 4.Kerr J. B., De Kretser D. M. 1974. Proceedings: the role of the Sertoli cell in phagocytosis of the residual bodies of spermatids. J. Reprod. Fertil. 36: 439–440. [DOI] [PubMed] [Google Scholar]
- 5.Poulos A., Johnson D. W., Beckman K., White I. G., Easton C. 1987. Occurrence of unusual molecular species of sphingomyelin containing 28-34-carbon polyenoic fatty acids in ram spermatozoa. Biochem. J. 248: 961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furland N. E., Zanetti S. R., Oresti G. M., Maldonado E. N., Aveldano M. I. 2007. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J. Biol. Chem. 282: 18141–18150. [DOI] [PubMed] [Google Scholar]
- 7.Poulos A., Sharp P., Johnson D., White I., Fellenberg A. 1986. The occurrence of polyenoic fatty acids with greater than 22 carbon atoms in mammalian spermatozoa. Biochem. J. 240: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furland N. E., Oresti G. M., Antollini S. S., Venturino A., Maldonado E. N., Aveldano M. I. 2007. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J. Biol. Chem. 282: 18151–18161. [DOI] [PubMed] [Google Scholar]
- 9.Robinson B. S., Johnson D. W., Poulos A. 1992. Novel molecular species of sphingomyelin containing 2-hydroxylated polyenoic very-long-chain fatty acids in mammalian testes and spermatozoa. J. Biol. Chem. 267: 1746–1751. [PubMed] [Google Scholar]
- 10.Oresti G. M., Ayuza-Aresti P. L., Gigola G., Reyes L. E., Aveldano M. I. 2010. Sequential depletion of rat testicular lipids with long-chain and very-long-chain polyenoic fatty acids after X-ray induced interruption of spermatogenesis. J. Lipid Res. Epub ahead of print. June 7, 2010; doi:10.1194/jlr.M006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman J. K., Gray M. E., Coniglio J. G. 1978. The lipid composition of isolated rat spermatids and spermatocytes. Biochim. Biophys. Acta. 530: 367–374. [DOI] [PubMed] [Google Scholar]
- 12.Grogan W. M., Farnham W. F., Szopiak B. A. 1981. Long chain polyenoic acid levels in viably sorted, highly enriched mouse testis cells. Lipids. 16: 401–410. [DOI] [PubMed] [Google Scholar]
- 13.Davis J. T., Bridges R. B., Coniglio J. G. 1966. Changes in lipid composition of the maturing rat testis. Biochem. J. 98: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprecher H., Chen Q., Yin F. Q. 1999. Regulation of the biosynthesis of 22:5n-6 and 22:6n-3: a complex intracellular process. Lipids. 34(Suppl): S153–S156. [DOI] [PubMed] [Google Scholar]
- 15.Leonard A. E., Kelder B., Bobik E. G., Chuang L. T., Lewis C. J., Kopchick J. J., Mukerji P., Huang Y. S. 2002. Identification and expression of mammalian long-chain PUFA elongation enzymes. Lipids. 37: 733–740. [DOI] [PubMed] [Google Scholar]
- 16.Furland N. E., Maldonado E. N., Aveldano M. I. 2003. Very long chain PUFA in murine testicular triglycerides and cholesterol esters. Lipids. 38: 73–80. [DOI] [PubMed] [Google Scholar]
- 17.Romrell L. J., Bellve A. R., Fawcett D. W. 1976. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev. Biol. 49: 119–131. [DOI] [PubMed] [Google Scholar]
- 18.Reyes J. G., Diaz A., Osses N., Opazo C., Benos D. J. 1997. On stage single cell identification of rat spermatogenic cells. Biol. Cell. 89: 53–66. [DOI] [PubMed] [Google Scholar]
- 19.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 20.Holub B. J., Skeaff C. M. 1987. Nutritional regulation of cellular phosphatidylinositol. Methods Enzymol. 141: 234–244. [DOI] [PubMed] [Google Scholar]
- 21.Rouser G., Fkeischer S., Yamamoto A. 1970. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5: 494–496. [DOI] [PubMed] [Google Scholar]
- 22.Christie W. W. 1982. Lipid Analysis. 2nd edition Pergamon Press, Oxford, UK. [Google Scholar]
- 23.Beckman J. K., Coniglio J. G. 1979. A comparative study of the lipid composition of isolated rat Sertoli and germinal cells. Lipids. 14: 262–267. [DOI] [PubMed] [Google Scholar]
- 24.Grogan W. M., Lam J. W. 1982. Fatty acid synthesis in isolated spermatocytes and spermatids of mouse testis. Lipids. 17: 604–611. [DOI] [PubMed] [Google Scholar]
- 25.Grogan W. M., Huth E. G. 1983. Biosynthesis of long-chain polyenoic acids from arachidonic acid in cultures of enriched spermatocytes and spermatids from mouse testis. Lipids. 18: 275–284. [DOI] [PubMed] [Google Scholar]
- 26.Retterstol K., Tran T. N., Haugen T. B., Christophersen B. O. 2001. Metabolism of very long chain polyunsaturated fatty acids in isolated rat germ cells. Lipids. 36: 601–606. [DOI] [PubMed] [Google Scholar]
- 27.Nenicu A., Luers G. H., Kovacs W., David M., Zimmer A., Bergmann M., Baumgart-Vogt E. 2007. Peroxisomes in human and mouse testis: differential expression of peroxisomal proteins in germ cells and distinct somatic cell types of the testis. Biol. Reprod. 77: 1060–1072. [DOI] [PubMed] [Google Scholar]
- 28.Rodemer C., Thai T. P., Brugger B., Kaercher T., Werner H., Nave K. A., Wieland F., Gorgas K., Just W. W. 2003. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 12: 1881–1895. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y., Nakagawa A., Nagaosa K., Shiratsuchi A., Nakanishi Y. 2002. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular sertoli cells. J. Biol. Chem. 277: 27559–27566. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa A., Nagaosa K., Hirose T., Tsuda K., Hasegawa K., Shiratsuchi A., Nakanishi Y. 2004. Expression and function of class B scavenger receptor type I on both apical and basolateral sides of the plasma membrane of polarized testicular Sertoli cells of the rat. Dev. Growth Differ. 46: 283–298. [DOI] [PubMed] [Google Scholar]
- 31.Saether T., Tran T. N., Rootwelt H., Christophersen B. O., Haugen T. B. 2003. Expression and regulation of delta5-desaturase, delta6-desaturase, stearoyl-coenzyme A (CoA) desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol. Reprod. 69: 117–124. [DOI] [PubMed] [Google Scholar]
- 32.Kabbaj O., Holm C., Vitale M. L., Pelletier R. M. 2001. Expression, activity, and subcellular localization of testicular hormone-sensitive lipase during postnatal development in the guinea pig. Biol. Reprod. 65: 601–612. [DOI] [PubMed] [Google Scholar]
- 33.Mizutani Y., Kihara A., Chiba H., Tojo H., Igarashi Y. 2008. 2-Hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J. Lipid Res. 49: 2356–2364. [DOI] [PubMed] [Google Scholar]
- 34.Rabionet M., van der Spoel A. C., Chuang C. C., von Tumpling-Radosta B., Litjens M., Bouwmeester D., Hellbusch C. C., Korner C., Wiegandt H., Gorgas K., et al. 2008. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J. Biol. Chem. 283: 13357–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckhardt M., Yaghootfam A., Fewou S. N., Zoller I., Gieselmann V. 2005. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 388: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida Y., Hama H., Alderson N. L., Douangpanya S., Wang Y., Crumrine D. A., Elias P. M., Holleran W. M. 2007. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J. Biol. Chem. 282: 13211–13219. [DOI] [PubMed] [Google Scholar]
- 37.Tafesse F. G., Ternes P., Holthuis J. C. 2006. The multigenic sphingomyelin synthase family. J. Biol. Chem. 281: 29421–29425. [DOI] [PubMed] [Google Scholar]
- 38.Tafesse F. G., Huitema K., Hermansson M., van der Poel S., van den Dikkenberg J., Uphoff A., Somerharju P., Holthuis J. C. M. 2007. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J. Biol. Chem. 282: 17537–17547. [DOI] [PubMed] [Google Scholar]
- 39.Lee N. P., Mruk D. D., Xia W., Cheng C. Y. 2007. Cellular localization of sphingomyelin synthase 2 in the seminiferous epithelium of adult rat testes. J. Endocrinol. 192: 17–32. [DOI] [PubMed] [Google Scholar]
- 40.Butler A., He X., Gordon R. E., Wu H. S., Gatt S., Schuchman E. H. 2002. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am. J. Pathol. 161: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler A., Gordon R. E., Gatt S., Schuchman E. H. 2007. Sperm abnormalities in heterozygous acid sphingomyelinase knockout mice reveal a novel approach for the prevention of genetic diseases. Am. J. Pathol. 170: 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otala M., Pentikainen M. O., Matikainen T., Suomalainen L., Hakala J. K., Perez G. I., Tenhunen M., Erkkila K., Kovanen P., Parvinen M., et al. 2005. Effects of acid sphingomyelinase deficiency on male germ cell development and programmed cell death. Biol. Reprod. 72: 86–96. [DOI] [PubMed] [Google Scholar]
- 43.Huyghe S., Schmalbruch H., De G. K., Verhoeven G., Guillou F., Van Veldhoven P. P., Baes M. 2006. Peroxisomal multifunctional protein 2 is essential for lipid homeostasis in Sertoli cells and male fertility in mice. Endocrinology. 147: 2228–2236. [DOI] [PubMed] [Google Scholar]
- 44.Fukasawa M., Atsuzawa K., Mizutani K., Nakazawa A., Usuda N. 2010. Immunohistochemical localization of mitochondrial fatty acid {beta}-oxidation enzymes in rat testis. J. Histochem. Cytochem. 58: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong W., Wang H., Wu H., Chen Y., Han D. 2009. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction. 137: 469–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.