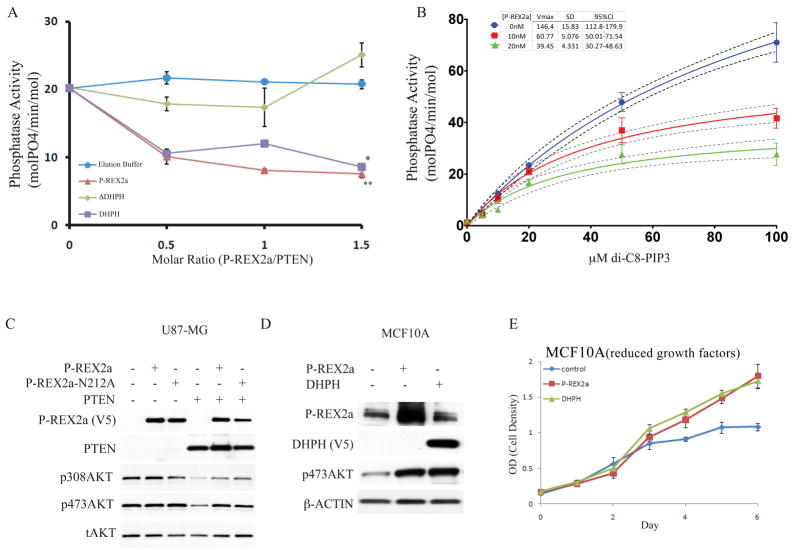
Fig. 2. Inhibition of PTEN phosphatase activity by P-REX2a.
(A) Full-length P-REX2a, or a deletion of the DHPH domain (ΔDHPH) or the DHPH domain alone were added in the indicated molar volumes to 40nM PTEN, purified from HEK293 cells, and phosphatase activity of PTEN was assayed with 20μM di-C8-PIP3. This is a representative experiment, error bars indicate standard deviation (n=3), **p<0.005, *p<0.05 by ANOVA. (B) P-REX2a (10nM or 20nM) was added to the reactions and phosphate released was measured over a range of di-C8-PIP3 using 40nM PTEN. Regression line (solid) to Michaelis-Menten kinetics is shown along with 95% confidence interval (dotted lines). Vmax values are shown in table along with standard deviation and 95% confidence interval. Representative experiment shown and error bars represent +/− standard deviation (n=3). (C) Effect of P-REX2a and a GEF dead point mutant of P-REX2a, N212A, on phosphorylation of AKT in presence and absence of PTEN. (D) Effect of expression of P-REX2a alone in MCF10A cells on abundance of p473AKT. The phosphorylation status of T308AKT was not detectable under normal growth conditions in MCF10A cells. (E) Effect of P-REX2a and DHPH on proliferation of MCF10A cells grown in reduced growth factors (0.1% serum).