Abstract
Familial hypercholesterolemia is associated with premature atherosclerosis. Since endothelial dysfunction is an early event in atherogenesis, we used a noninvasive method to assess endothelial function in the systemic arteries of 30 children aged 7-17 yr (median 11) with familial hypercholesterolemia (2 homozygotes, 28 heterozygotes, total cholesterol 240-696 mg/dl) and 30 healthy age- and sex-matched controls. Using high resolution ultrasound, the diameter of the superficial femoral artery was measured at rest, in response to reactive hyperemia (with increased flow causing endothelium-dependent dilation), and after sublingual glyceryltrinitrate (causing endothelium-independent vasodilation). Flow-mediated dilation was present in the controls (7.5 +/- 0.7%) but was impaired or absent in the hypercholesterolemic children (1.2 +/- 0.4%, P < 0.0001). Total cholesterol was inversely correlated with flow-mediated dilation (r = -0.61, P < 0.0001). In the hypercholesterolemic children, flow-mediated dilation was inversely related to the lipoprotein(a) level (r = -0.61, P = 0.027) but not to other lipid fractions. Glyceryltrinitrate-induced dilation was present in all subjects but was lower in the hypercholesterolemia group (10.0 +/- 0.6% vs 12.4 +/- 0.8%, P = 0.023). Thus, impaired endothelium-dependent dilation is present in children with familial hypercholesterolemia as young as 7 yr of age and the degree of impairment is related to the lipoprotein(a) level.
Full text
PDF
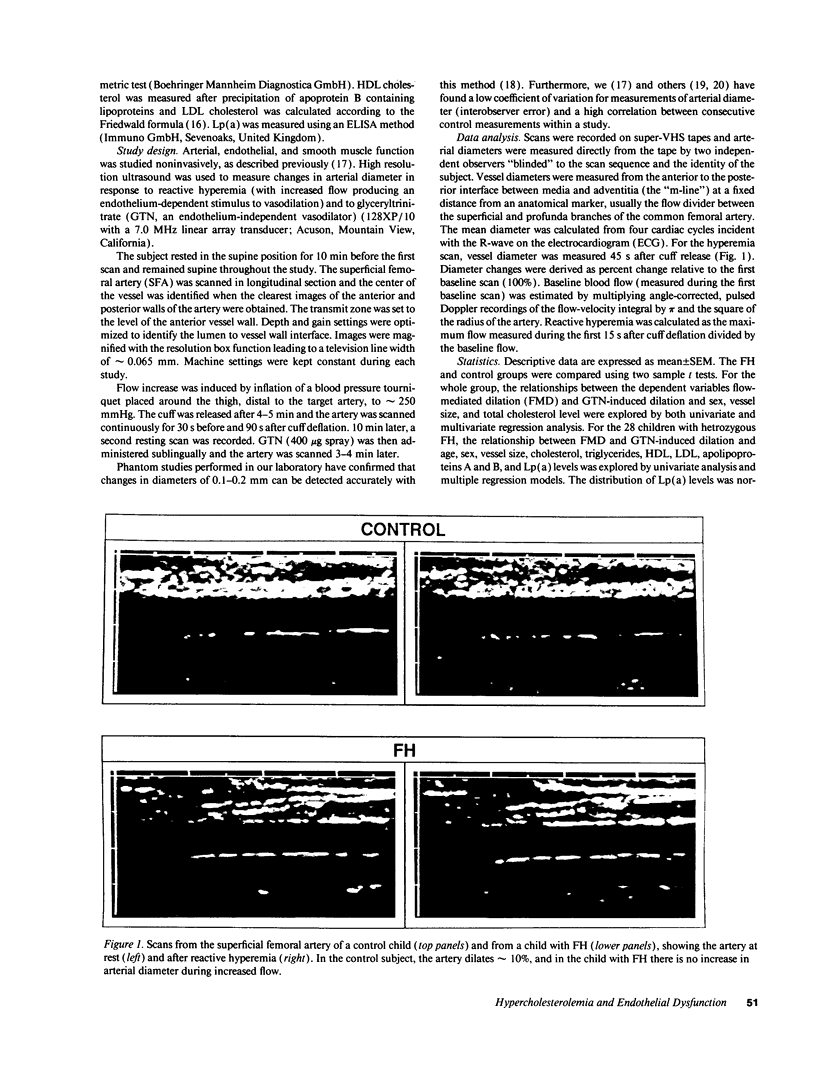
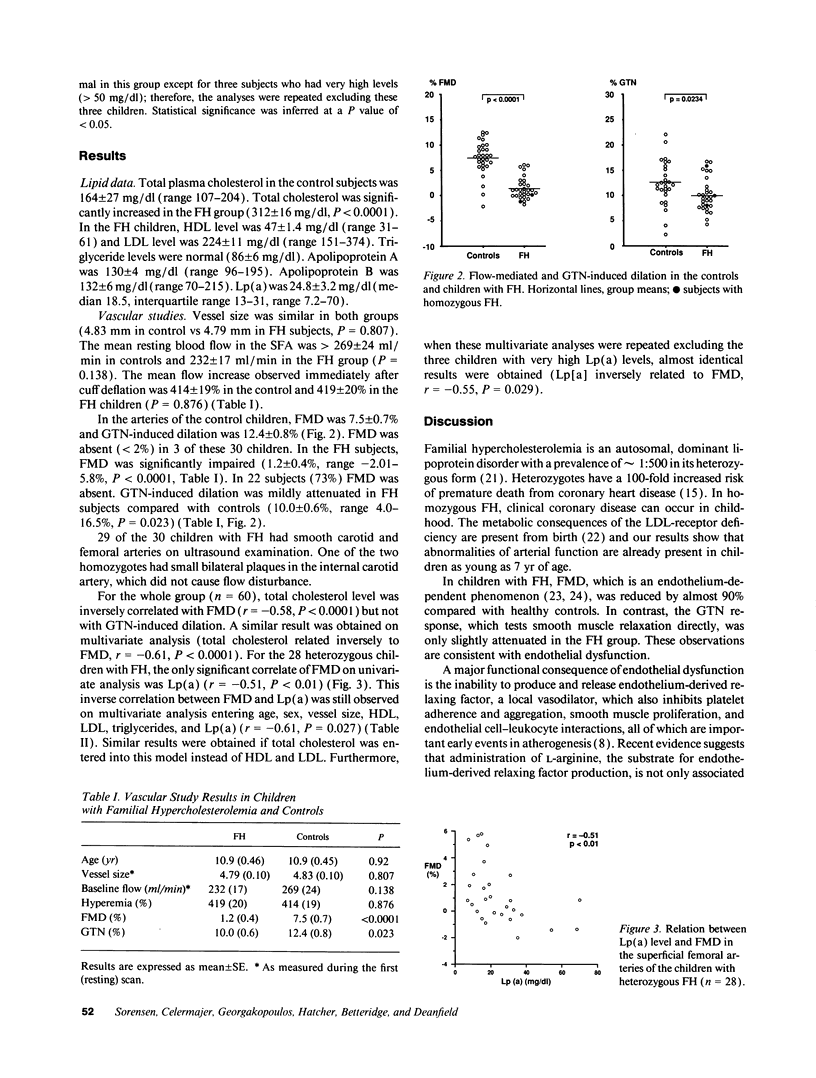



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. A., Mark A. L. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989 Jan;79(1):93–100. doi: 10.1161/01.cir.79.1.93. [DOI] [PubMed] [Google Scholar]
- Armstrong V. W., Cremer P., Eberle E., Manke A., Schulze F., Wieland H., Kreuzer H., Seidel D. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis. 1986 Dec;62(3):249–257. doi: 10.1016/0021-9150(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Blankenhorn D. H., Azen S. P., Crawford D. W., Nessim S. A., Sanmarco M. E., Selzer R. H., Shircore A. M., Wickham E. C. Effects of colestipol-niacin therapy on human femoral atherosclerosis. Circulation. 1991 Feb;83(2):438–447. doi: 10.1161/01.cir.83.2.438. [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Gooch V. M., Spiegelhalter D. J., Miller O. I., Sullivan I. D., Lloyd J. K., Deanfield J. E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992 Nov 7;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K., Ryalls M., Robinson J., Thomas O., Leonard J. V., Deanfield J. E. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol. 1993 Sep;22(3):854–858. doi: 10.1016/0735-1097(93)90203-d. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Zitnay K. M., Haudenschild C. C., Cunningham L. D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988 Nov;63(5):903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Singer A. H., Tsao P., Zera P., Rowan R. A., Billingham M. E. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992 Sep;90(3):1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H., Zeiher A. M., Meinzer K., Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991 Dec 21;338(8782-8783):1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- ENOS W. F., HOLMES R. H., BEYER J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc. 1953 Jul 18;152(12):1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Fless G. M., Kohr W. J., McLean J. W., Xu Q. T., Miller C. G., Lawn R. M., Scanu A. M. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc Natl Acad Sci U S A. 1987 May;84(10):3224–3228. doi: 10.1073/pnas.84.10.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish R. D., Nabel E. G., Selwyn A. P., Ludmer P. L., Mudge G. H., Kirshenbaum J. M., Schoen F. J., Alexander R. W., Ganz P. Responses of coronary arteries of cardiac transplant patients to acetylcholine. J Clin Invest. 1988 Jan;81(1):21–31. doi: 10.1172/JCI113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- Galle J., Bassenge E., Busse R. Oxidized low density lipoproteins potentiate vasoconstrictions to various agonists by direct interaction with vascular smooth muscle. Circ Res. 1990 May;66(5):1287–1293. doi: 10.1161/01.res.66.5.1287. [DOI] [PubMed] [Google Scholar]
- Genest J., Jr, McNamara J. R., Ordovas J. M., Jenner J. L., Silberman S. R., Anderson K. M., Wilson P. W., Salem D. N., Schaefer E. J. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J Am Coll Cardiol. 1992 Mar 15;19(4):792–802. doi: 10.1016/0735-1097(92)90520-w. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Armstrong M. L., Freiman P. C., Heistad D. D. Restoration of endothelium-dependent relaxation by dietary treatment of atherosclerosis. J Clin Invest. 1987 Dec;80(6):1808–1811. doi: 10.1172/JCI113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. H. St Cyres lecture. Endothelium in control. Br Heart J. 1991 Mar;65(3):116–125. doi: 10.1136/hrt.65.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake D. B., Stein E. A., Dujovne C. A., Harris W. S., Feldman E. B., Miller V. T., Tobert J. A., Laskarzewski P. M., Quiter E., Held J. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med. 1993 Apr 29;328(17):1213–1219. doi: 10.1056/NEJM199304293281701. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Plane F., Bruckdorfer K. R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol. 1990 May;100(1):21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B. Hypertension, blood lipids, and cigarette smoking as co-risk factors for coronary heart disease. Ann N Y Acad Sci. 1978 Mar 30;304:128–139. doi: 10.1111/j.1749-6632.1978.tb25585.x. [DOI] [PubMed] [Google Scholar]
- Kimm S. Y., Payne G. H., Lakatos E., Webber L. S., Greenblatt J. Primary care physicians and children's blood cholesterol. Prev Med. 1992 Mar;21(2):191–202. doi: 10.1016/0091-7435(92)90018-d. [DOI] [PubMed] [Google Scholar]
- Klag M. J., Ford D. E., Mead L. A., He J., Whelton P. K., Liang K. Y., Levine D. M. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med. 1993 Feb 4;328(5):313–318. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]
- Komori K., Shimokawa H., Vanhoutte P. M. Hypercholesterolemia impairs endothelium-dependent relaxations to aggregating platelets in porcine iliac arteries. J Vasc Surg. 1989 Sep;10(3):318–325. [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Kwiterovich P. O., Jr Diagnosis and management of familial dyslipoproteinemia in children and adolescents. Pediatr Clin North Am. 1990 Dec;37(6):1489–1523. doi: 10.1016/s0031-3955(16)37021-3. [DOI] [PubMed] [Google Scholar]
- Leung W. H., Lau C. P., Wong C. K. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolaemic patients. Lancet. 1993 Jun 12;341(8859):1496–1500. doi: 10.1016/0140-6736(93)90634-s. [DOI] [PubMed] [Google Scholar]
- Liao J. K., Bettmann M. A., Sandor T., Tucker J. I., Coleman S. M., Creager M. A. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991 Apr;68(4):1027–1034. doi: 10.1161/01.res.68.4.1027. [DOI] [PubMed] [Google Scholar]
- Lichtlen P. R., Nikutta P., Jost S., Deckers J., Wiese B., Rafflenbeul W. Anatomical progression of coronary artery disease in humans as seen by prospective, repeated, quantitated coronary angiography. Relation to clinical events and risk factors. The INTACT Study Group. Circulation. 1992 Sep;86(3):828–838. doi: 10.1161/01.cir.86.3.828. [DOI] [PubMed] [Google Scholar]
- McLenachan J. M., Vita J., Fish D. R., Treasure C. B., Cox D. A., Ganz P., Selwyn A. P. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation. 1990 Oct;82(4):1169–1173. doi: 10.1161/01.cir.82.4.1169. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Newman T. B., Browner W. S., Hulley S. B. Childhood cholesterol screening: contraindicated. JAMA. 1992 Jan 1;267(1):100–102. [PubMed] [Google Scholar]
- Olsson A. G. Regression of femoral atherosclerosis. Circulation. 1991 Feb;83(2):698–700. doi: 10.1161/01.cir.83.2.698. [DOI] [PubMed] [Google Scholar]
- Pohl U., Holtz J., Busse R., Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986 Jan;8(1):37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Salonen R., Salonen J. T. Determinants of carotid intima-media thickness: a population-based ultrasonography study in eastern Finnish men. J Intern Med. 1991 Mar;229(3):225–231. doi: 10.1111/j.1365-2796.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Sandholzer C., Boerwinkle E., Saha N., Tong M. C., Utermann G. Apolipoprotein(a) phenotypes, Lp(a) concentration and plasma lipid levels in relation to coronary heart disease in a Chinese population: evidence for the role of the apo(a) gene in coronary heart disease. J Clin Invest. 1992 Mar;89(3):1040–1046. doi: 10.1172/JCI115645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M. Lipoprotein(a). A genetic risk factor for premature coronary heart disease. JAMA. 1992 Jun 24;267(24):3326–3329. doi: 10.1001/jama.267.24.3326. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L., Sparks H. V. Response of large and small coronary arteries to nitroglycerin, NaNO 2 , and adenosine. Am J Physiol. 1972 Jul;223(1):223–228. doi: 10.1152/ajplegacy.1972.223.1.223. [DOI] [PubMed] [Google Scholar]
- Seed M., Hoppichler F., Reaveley D., McCarthy S., Thompson G. R., Boerwinkle E., Utermann G. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990 May 24;322(21):1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- Simon B. C., Cunningham L. D., Cohen R. A. Oxidized low density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J Clin Invest. 1990 Jul;86(1):75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg L. A., Strong J. P. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983 May-Jun;3(3):187–198. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- Tomita T., Ezaki M., Miwa M., Nakamura K., Inoue Y. Rapid and reversible inhibition by low density lipoprotein of the endothelium-dependent relaxation to hemostatic substances in porcine coronary arteries. Heat and acid labile factors in low density lipoprotein mediate the inhibition. Circ Res. 1990 Jan;66(1):18–27. doi: 10.1161/01.res.66.1.18. [DOI] [PubMed] [Google Scholar]
- Vita J. A., Treasure C. B., Nabel E. G., McLenachan J. M., Fish R. D., Yeung A. C., Vekshtein V. I., Selwyn A. P., Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990 Feb;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Wendelhag I., Wiklund O., Wikstrand J. Arterial wall thickness in familial hypercholesterolemia. Ultrasound measurement of intima-media thickness in the common carotid artery. Arterioscler Thromb. 1992 Jan;12(1):70–77. doi: 10.1161/01.atv.12.1.70. [DOI] [PubMed] [Google Scholar]
- Wines P. A., Schmitz J. M., Pfister S. L., Clubb F. J., Jr, Buja L. M., Willerson J. T., Campbell W. B. Augmented vasoconstrictor responses to serotonin precede development of atherosclerosis in aorta of WHHL rabbit. Arteriosclerosis. 1989 Mar-Apr;9(2):195–202. doi: 10.1161/01.atv.9.2.195. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Bossaller C., Cartwright J., Jr, Henry P. D. Videomicroscopic demonstration of defective cholinergic arteriolar vasodilation in atherosclerotic rabbit. J Clin Invest. 1988 Jun;81(6):1752–1758. doi: 10.1172/JCI113516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991 Feb;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]




